Airborne and animal transmission of disease in pig herds
Swine diseases can be spread via a number of avenues, making biosecurity a priority, especially in outdoor pig herds where birds and insects can carry diseases from other farms into naive herds.If the organism is exhaled from the pig in large droplets, as is common with respiratory bacterial infections, then the actual spread of the organism is limited to probably no more than 50 metres and field studies suggest it is often less than 5 metres. Conversely, some viruses which are extremely small have been shown to be carried by wind for many kilometres under ideal conditions. For example foot-and-mouth disease virus was shown to have been windborne over land 20km (12 miles) and an incredible 300km (190 miles) over water. Aujeszky's virus has been carried 9km (6 miles) over land.
Diseases/organisms spread through the air by aerosol droplets
Short distances (i.e. a few metres)
- Actinobacillus pleuropneumoniae that causes severe haemorrhagic and necrotic pneumonia.
- Toxigenic Pasteurella multocidia that causes atrophic rhinitis.
- Mycoplasma hyopneumoniae that causes enzootic pneumonia. This can carry 2km.
- Haemophilus parasuis that causes glässers disease.
- Mycoplasma hyosynoviae that causes arthritis.
- Other pasteurella that are involved in pneumonia.
- Streptococcus suis that causes meningitis and other conditions.
Intermediate distances up to about 3km (2 miles)
- Enzootic pneumonia.
- Influenza, probably unproven.
- Porcine reproductive and respiratory syndrome (PRRS) virus.
- Porcine respiratory coronavirus (PRCV).
Relatively long distances >9km (6 miles)
- Aujeszky's disease.
- Foot-and-mouth disease.
Not all viruses become windborne. The viruses of TGE, porcine epidemic diarrhoea, porcine parvovirus, classical swine fever, African swine fever, encephalomyocarditis, or swine vesicular disease are not known to spread on the wind. Theoretically one would expect that viruses causing respiratory infections would be most likely to be spread on wind.
Bacterial infections are less likely to be carried so far on the wind because of the larger size of the droplets. Furthermore it has been shown that the great majority of airborne bacteria in pig farms are dead (although their endotoxins can still cause problems). Nevertheless there is strong evidence that Mycoplasma hyopneumoniae which causes enzootic pneumonia (EP) sometimes travels at least 3km (2 miles). Field experiences have shown that if another pig farm with EP is clearly visible from a newly repopulated pig farm which does not have EP, then sooner or later the healthy pig farm will break down with the disease. There is little firm evidence about the ability or frequency of the other bacterial infections being windborne.
The frustration about windborne infection is that you have no defence against it except in the choice of the location in which you build your pig farm. Keeping pigs in totally enclosed buildings is no defence. If the ventilation involves fans the air inlets act like vacuum cleaners. Filters fine enough to filter micro-organisms are possible but are impractical.
In hot dry summers the chance of aerosol spread between farms is low, but this does not always hold true for the same regions in winter, particularly at night. It is not surprising that epidemics of FMD occurred in winter.
Transmission by birds
The three porcine diseases that are definitely transmitted by birds are avian tuberculosis. transmissible gastro-enteritis (TGE) and erysipelas, although it is likely that other infectious agents such as PRRS virus may be carried on birds' feet or pass through their alimentary tracts into their droppings.
Birds visit pig farms mainly in winter time, late fall or early spring, to eat pig feed. Pathogenic organisms on their feet could conceivably contaminate the feed. More important, their droppings contaminate the feed, the floors, and sometimes stored bedding, such as straw or shavings. In temperate and warm climates, pig buildings are often open on one or more sides. Bird proofing then involves extensive netting which is not often done in spite of the risk from birds. The opinion of farmers in England who have done this claim that it is cost effective in the savings made in feed alone. In extreme climates pig buildings are usually totally enclosed so the risk from birds is much reduced. Even then, there is still the possibility of birds defecating on stored or spilt food or stored bedding outside the buildings, the organisms in the faeces being carried in to the pigs on people's feet as well as on the bedding or feed.
In many countries the most dangerous birds are starlings. They tend to travel in large flocks, flying in a 30km (19 mile) radius and landing on numerous different pig farms in a day. In a study carried out it was estimated that about 30% of new outbreaks of TGE were due to starlings. When starlings ingest TGE virus, which is easily done when they are feeding on a pig farm during the acute stage of a TGE outbreak, they shed it in their droppings for up to 36 hours. There appears to be no evidence that porcine epidemic diarrhoea (PED) is spread by starlings but it would not be surprising if it were. Sea gulls have also been implicated in the spread of TGE in the UK. Porcine reproductive and respiratory syndrome (PRRS) virus has been shown to multiply in ducks but no work has been done to show whether it can multiply in other birds.
Birds with avian tuberculosis shed vast numbers of Mycobacterium avium in their droppings, which, when ingested by pigs cause typical tubercle lesions in the lymph nodes of the neck and mesentery, resulting in condemnation of the head and offal at slaughter. Sometimes individual herds suffer long periods with high condemnation rates. Bird-proofing the pig farm and removing all the stored bedding in some cases may not have the desired effect. It must be remembered that Mycobacterium avium is not a uniform species of bacterium but covers a wide spectrum of variants some of which multiply readily saprophytically (outside the pig). Some strains multiply, for example in peat used for bedding or in water tanks.
Birds are sometimes blamed for outbreaks of erysipelas in pigs. They can become infected by the causal organism, and may then shed it in their droppings. Most outbreaks however originate from the pigs themselves. The organism resides in the tonsils sub-clinically for long periods and becomes endemic in herds. For reasons that are not clear, probably related to stress, an individual carrier may develop clinical signs and then shed large numbers of virulent organism in the faeces.
Birds have been incriminated in the spread of diseases such as FMD and salmonella (and recently in the UK of PRRS) but it has rarely if ever been proved. Salmonellae can be carried and shed by birds in their droppings but often the serotypes are not those that would harm pigs. Highly infectious agents such as FMD could be carried for short periods mechanically on birds' feet but there is no firm evidence that this has played a significant role in spread. Sea gulls, may be involved in the carriage of materials contaminated by pig products, such as pig-meat wrappings from garbage dumps, into pig farms. (The wind may also blow such material into a pig farm if the garbage dump is nearby).
Transmission by flies
Flies are common on pig farms and have access to contaminated materials such as dead pigs, the secretions and excretions of diseased pigs and faeces. They frequently travel up to 2-3km (1-2 miles) between pig farms in breezy weather particularly in summer time and are attracted by smells that are slightly out of the line of the wind direction. Studies carried out in Cambridge showed that when the common house fly (Musca domestica) was fed on materials contaminated with Streptococcus suis type 2 the organism remained viable in the fly, probably in its crop, for up to five days and it would then contaminate whatever it fed on. Apparently, before feeding, the house fly tends to vomit its crop contents. Flies have also been shown to carry other infections, including Brachyspira hyodysenteriae, the cause of swine dysentery. It seems reasonable to assume that they can carry many more infections than those that have been studied and reported. However, we should be wary about concluding that because they can carry an organism they necessarily play a role in its spread, The dose of organism that they carry may be extremely small and in some cases, such as Brachyspira hyodysenteriae, may be below the infective dose required to establish the infection in a pig. Clinical observations suggest they play a role in disease in farrowing houses.
Transmission by rats and mice
What pig disease might they carry? It has been shown that Brachyspira hyodysenteriae, the causal organism of swine dysentery, can infect mice and can be maintained in mouse colonies in pig farms. B. hyodysenteriae, isolated from mice on infected pig farms, has been shown to be pathogenic for pigs. Mice may provide one explanation why some pig farms break down with swine dysentery after they have been repopulated with clean stock or after attempts at eradication by blanket medication have been carried out. Although rats have been infected experimentally with B. hyodysenteriae, they are not thought to play any role in its spread in the field.
The rat is the natural host of the virus of encephalomyocarditis which is implicated in outbreaks of so called SMEDI (stillbirths, mummification, embryonic deaths and infertility) in the USA. It is thought that outbreaks occur in localised areas when rats are allowed to build up in numbers. In some countries the virus causes myocarditis and sudden death in pigs and other animals (e.g. zoo animals). In Cuba, this form of the disease is regarded as a major problem in pigs and has also caused deaths in apes, porcupines and cattle,
Rats and mice also carry and shed Salmonella typhimurium and other salmonella serotypes which affect pigs and dermatophytes, such as Trichophytum mentagrophytes which cause ringworm in pigs but fortunately rarely. Rats seem to be resistant to infection with Actinobacillus pleuropneumoniae and Streptococcus suis type 2. Mice can be infected experimentally by large doses of S. suis type 2 but there is no evidence that they carry the organism under field conditions.
House-mice remain resident in piggeries and do not normally travel between them. Field mice may travel short distances but are not likely to be significant in the spread of pig disease. Rats are much more mobile. Individuals will frequently cover 1-2km (1/2 to 1 mile) in a night. However their movement between pig farms depends on a complex social relationship in the resident rat communities in farm buildings. Both rats and mice may be carried inadvertently between farms in vehicles such as feed trucks.
Other wildlife
The wild boar is a vector of pig disease, particularly in Germany at the time of writing, classical swine fever (hog cholera) has been found to be endemic in the wild boar population and contaminates domestic pig herds. The wild boar in Africa was, of course, the source of African swine fever to domestic pigs farms in Africa and subsequently to Portugal and Spain, (and to other countries).
Lawsonia intracellularis bacterium has been identified as the cause of porcine enteropathy and it has been transmitted experimentally to hamsters and mice. Porcine enteropathies in which the same antigen has been detected have also been seen in ferrets and rabbits. It seems likely therefore that this organism could be spread by a variety of other species and it is not surprising that the disease syndrome occurs sometimes in the most secure highest health herds.
A range of leptospira serovars are carried by wild animals but most are not normally pathogenic to pigs. Individual pigs may become ill with Leptospira icterohaemorrhagiae from rats. A main pig pathogenic serovar in North America and some other parts of the world (not the UK or Ireland) L. pomona is shed by hedgehogs and possibly mice and rats. Other serovars which affect pigs are present in wildlife in other countries however a serovar which is pathogenic in one country may not be pathogenic in other countries. The presence of Brucella suis in wild hares in Denmark and France has contaminated outdoor herds.
Domesticated/farm animals
What is the risk of keeping herds of high health status pigs near other farm livestock including poultry, or of keeping dogs and cats in such herds? In countries which do not have FMD, the risk in practice seems to be low. One can theorise about the contamination with salmonellae from calves, of toxigenic Pasteurella multocidia from cattle and sheep, of Actinobacillus pleuropneumoniae from cattle, sheep and deer, of Erysipelothrix from poultry and Streptococcus suis (various serotypes) from cattle, sheep, goats or horses. In practice, provided they are physically separated from each other, there is little risk. However, outbreaks of disease caused by some of these organisms sometimes occur without obvious explanation. The source might be other animals. Aujeszky's disease (pseudorabies) crosses species but the risk is mainly the other way around, (i.e. from the pigs to the cattle and dogs).
Dogs can shed the virus of TGE for up to 14 days after eating contaminated material such as dead piglets. This is clearly a risk when a nearby neighbour gets TGE but farm dogs do not usually wander great distances and there are so many other ways in which TGE can be spread between neighbouring herds that the importance of the dog as a common vector is probably small. Farm dogs have also been shown to carry B. hyodysenteriae but it is difficult to assess whether or not they play any role in the spread of swine dysentery . Recent studies have shown that Leptospira bratislava can be shed in dogs' urine. If the dog is a good watch dog then its benefits in keeping intruders and other animals at bay may outweigh its risk of introducing TGE, swine dysentery or leptospirosis. Pig farmers in regions where classical swine fever (hog cholera), African swine fever and FMD occur should be a little more careful about dogs because of the danger of carrying bones into the farm
Cats in pig farms are frowned upon in the UK. The reason is often the risk of atrophic rhinitis, but in the many SPF herds in Denmark, cats are kept routinely to control mice and rats. If they stray from the premises they are killed or not allowed to return. Atrophic rhinitis is not a common cause of breakdown in the large Danish SPF programme. The main cause of breakdowns is enzootic pneumonia with Actinobacillus pleuropneumoniae also a problem.
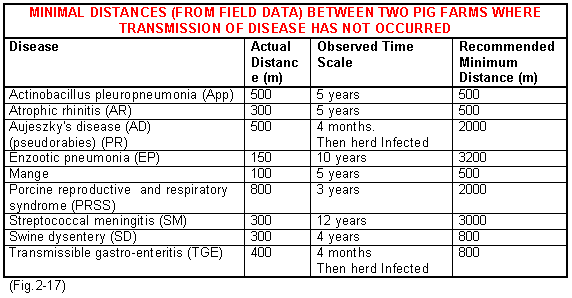
The two greatest risks of contamination to your herd are neighbouring infected pig herds and the introduction of disease through the purchased pig. It is always difficult to quantify the risk from neighbouring herds but Fig.2-17 shows the results of a field study looking at the minimum distances between herds when disease did not spread and the length of time observed. These results demonstrate minimum distances over which specific infections appear not to have travelled but they should not be used as a basis for a decision in practice. The recommended given distances should be used as a minimum for decision making purposes. If high health herds are being established a distance of at least 3.2km (2 miles) from other pigs is advised. However, the other factors must also be taken in to account.
Enzootic pneumonia, flu and PRRS are the three most difficult diseases to remain free from because they are windborne and are extremely common and widespread in the pig populations of most countries. The transfer of airborne mycoplasma is high within 800 metres of infected pigs but is reduced to almost negligible proportions provided there are no other sources of infected pigs within a 3.2 km radius (2 miles). The respective sizes of the two farms and their distance apart must also be considered. A 50 sow herd producing weaners is much less risk likely to spread infection than a 1000 sow herd of feeder pigs because of the reduced numbers of infectious particles produced by the smaller weaner only population. Another factor is the type of terrain in which herds are located, flat treeless countryside is worst, trees break up aerosol plumes.