Applications for New Vet Medicines Doubled in 2013
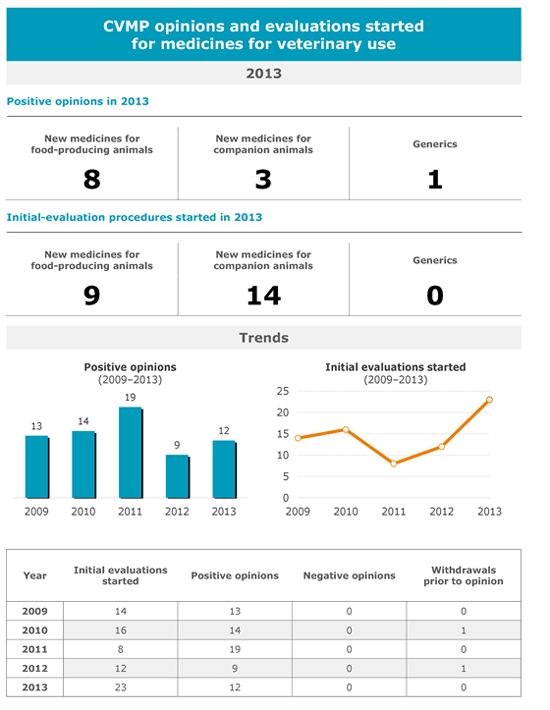
EU - In 2013, the European Medicines Agency’s (EMA) Committee for Medicinal Products for Veterinary Use started the evaluation of a record number of 23 marketing-authorisation applications for new veterinary medicines, almost twice the volume in 2012 (12). The majority of applications (16) are for immunologicals.The growing interest in the development of new veterinary medicines also seems to be demonstrated by the increasing number of scientific advice requests. In 2013, the CVMP received 40 requests from industry for scientific advice and responded to 34, which represents a slight increase compared with previous years (28 requests received in 2012). Similarly to medicines for human use, scientific advice in the veterinary area is designed to facilitate the development and access to the market of high-quality, effective and acceptably safe medicines.
In 2013, the CVMP issued 12 positive opinions for new veterinary medicines, including the first vaccine against foot-and-mouth disease for authorisation at European Union (EU) level. This is also the first vaccine using the full multistrain-dossier approach. This concept was established in 2010 to allow the swift availability of suitable vaccines in case of outbreaks of major livestock diseases that are caused by highly variable viruses, such as foot-and-mouth disease. Authorisation at EU level was also granted for a second vaccine against West Nile virus, a disease which is transmissible to man and has the potential to cause epidemics in horses since it is transmitted by infected mosquitos.
In the area of companion animals, five out of the eight medicines that received a positive opinion were products against parasites for dogs and cats, showing the attractive nature of this market for animal health companies.
The CVMP also recommended minor-use-minor-species (MUMS) classification for 20 veterinary products of which 10 qualified for financial incentives. This classification is intended to stimulate the development of new veterinary medicines for minor species and for rare diseases in major species that would otherwise not be developed under current market conditions.
Since its introduction in October 2010, this policy has been successful in terms of increasing interest from the animal-health industry in submitting applications for MUMS products, with over 70 products classified so far. Following an analysis of the requests received and incentives granted, the Agency has limited access to financial incentives offered under this policy since 1 September 2013 to MUMS products indicated for food-producing species on the basis that this type of product has the potential to be of most benefit to animal or public health. The significant benefits in terms of access to specific data requirements continue to be available for all types of products classified by CVMP as MUMS.