



EU Registration - A Worldwide Quality Benchmark
EU - The EU registration for mycotoxin deactivation products is not only the legal basis for official mycotoxin claims, according to Biomin. It is also a detailed evaluation with high standards for the efficacy and safety of a product.
Why does this make a difference?
Until 2009, there was no legislation in place recognizing feed additives with mycotoxin counteracting properties. As a result, more than 100 mycotoxin deactivation products available in the market were sold under non-mycotoxin specific claims, such as anti-caking agents. In 2010, after the EU introduced a new functional group of feed additives to recognize mycotoxin deactivation capabilities in products[1], BIOMIN submitted the first dossier.
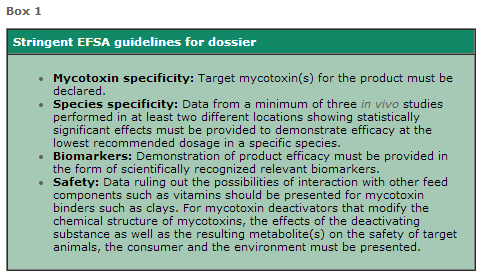
Submitting a dossier for mycotoxin deactivation products requires a comprehensive number of in vitro and in vivo experiments (Box 1). The stringent guidelines effectively discouraged many manufacturers from having their anti-mycotoxin additives legally authorized. This is where BIOMIN differs.
Because of its long-standing focus on mycotoxin research, BIOMIN was able to provide all the trials and experiments needed for the successful authorization of Mycofix® Secure for pigs, poultry and ruminants and Biomin® BBSH 797 for pigs. For more than two decades, BIOMIN has had its own research center working on creative and targeted solutions for mycotoxin deactivation and developing strong relationships with the mycotoxin research community globally.
[1] Regulation (EC) No 386/2009
The need for biomarkers
Apart from the laborious and costly experiments required to confirm the safety of a product, companies face the further challenge of developing and accomplishing biomarker studies that can directly prove the deactivation of mycotoxins in vivo (Box 2).
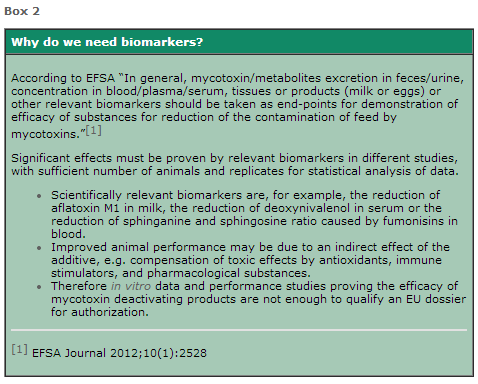
Most studies for mycotoxin deactivation products are performance studies trying to prove the mitigation of the harmful effects of mycotoxins but not the claimed deactivation of the toxin itself. To date, BIOMIN is the only company that has successfully proven the deactivation of mycotoxins with biomarkers.
Biomarker studies are quite difficult to accomplish. Most laboratories already fail to establish a validated analytical detection of mycotoxins in blood, urine or feces, where very sensitive and precise methods are needed. Conducting representative feeding trials and evaluating biomarkers requires advanced scientific expertise.
Bacteria and bentonite
The final authorization of Mycofix® Secure and Biomin® BBSH 797 is issued by the EU as non-holder specific authorization[1]. ‘Non-holder specific’ means that a product, fulfilling the criteria of the regulation, is allowed to claim the capability to deactivate a specific mycotoxin, independent of the company that submitted the dossier.
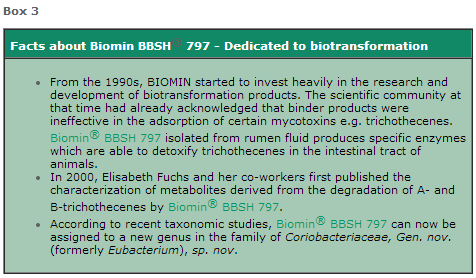
No other company can legitimately sell the unique trichothecene-detoxifying bacteria Biomin® BBSH 797, as BIOMIN is the sole patent holder.
Only BIOMIN is allowed to use the claim ‘deoxynivalenol biotransformation’, unless another company files its own dossier and receives authorization with its own strain supporting this claim (Box3).
It is different in the case of bentonite: The EU regulation[2] legalizing bentonite for aflatoxin deactivation is based on the dossier submitted by BIOMIN on its specific bentonite solely included in the Mycofix® product line. Any company selling bentonite fulfilling the criteria[3] is now allowed to sell the product “registered for mycotoxin deactivation (1m)” without submitting its own dossier.
[1] Regulation (EC) No 1016/2013
[2] Regulation (EC) No 1060/2013
[3] Regulation (EC) No 1060/2013 main criteria: smectite (dioctahedral montmorillonite) content ≥70 per cent and aflatoxin B1 binding capacity above 90 per cent in a buffer solution at pH 5.0, with 4mg/l aflatoxin B1 and 0.02 per cent feed additive
Aflatoxin-binding claim
Which official authority approves the fulfillment of the criteria of the regulation? In the case of any non-BIOMIN bentonite, no evaluation is required by the European Feed Safety Authority (EFSA) with regard to the identity, safety and efficacy of the product before it can be placed on the market under EU regulation 1060/2013. The claim “aflatoxin-binding” is allowed only for products that fulfill the main criteria3. To date, the majority of products in the market do not meet the criteria. Aflatoxin-binding claims made without the right data in place are considered illegal in the EU, and offending parties may face legal action.
Not all bentonites are equal
Bentonite is a natural clay and differs largely depending on the origin. Only the specific bentonite sold exclusively in the Mycofix® product line has undergone the complete EFSA procedure with all experiments and trials for identity, safety and efficacy and succeeded in a final authorization.
BIOMIN is responsible for legalizing the aflatoxin-binding claim of bentonites and the biotransformation of trichothecenes by Biomin® BBSH 797 in the European market.
Till now, BIOMIN is the only company to have received the authorization of the dossiers submitted for mycotoxin deactivation products. This authorization, which comes with strict and rigid requirements in the EU, helps customers to comprehensively compare products and make informed decisions with the scientific assurance of quality.






