



Vaccination protects against new emerging PRRS strain
Ingelvac PRRS® MLV provides cross-protection against a PRRSv 1-7-4 challenge.Boehringer Ingelheim (BI) is committed to demonstrating that Ingelvac PRRS® MLV vaccine provides protection against new, emerging strains of PRRSv. Previous studies have demonstrated that Ingelvac PRRS MLV provides protection against a number of heterologous PRRSv challenge isolates.1 More recently, we conducted a cross-protection study against the new PRRSv 1-7-4 isolate. The study demonstrated that Ingelvac PRRS MLV provided protection against this new strain of PRRSv.
Testing vaccine efficacy
In the study, pigs vaccinated with Ingelvac PRRS MLV demonstrated significant protection against a virulent PRRSv 1-7-4 challenge.2 Compared to non-vaccinated challenge-control pigs, Ingelvac PRRS MLV-vaccinated pigs demonstrated a significant reduction in lung lesions, a significant reduction in viremia, and a significant improvement in average daily weight gain (ADWG) following challenge. In addition, when compared to Fostera® PRRS-vaccinated pigs, Ingelvac PRRS MLV–vaccinated pigs demonstrated a numerical advantage in ADWG, and a significant difference in the reduction of the level of viremia at 14 days post challenge.
In this study, three week old pigs were randomised into groups and intramuscularly vaccinated with 2 mL of a placebo (non-vaccinated challenge controls), Ingelvac PRRS MLV or Fostera PRRS (day 0 of the study). Pigs were housed in rooms by group during the vaccination period. On day 28 of the study (D28), all pigs were commingled and challenged with 2.0 mL intramuscularly and 2.0 mL intranasally with 104.6 TCID50/mL of PRRSv 1-7-4. Serum samples, weights and temperatures were collected periodically from D0 through termination of the study on D42. On D42 (14 days post challenge), all pigs were necropsied, and lungs were scored for the presence of macroscopic lesions.
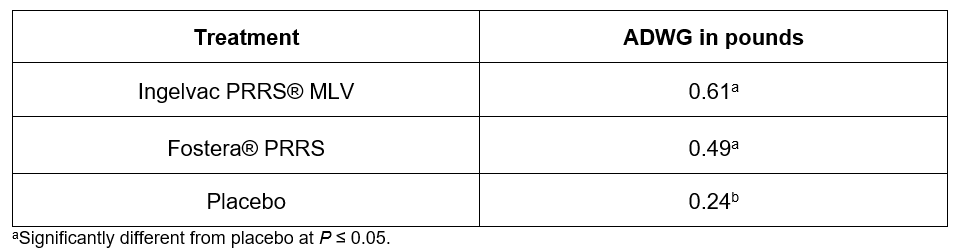
Pigs vaccinated with Ingelvac PRRS MLV had the lowest median percent lung lesion scores at 8.4 percent, and the highest ADWG (0.61 lb.) during the post-challenge period. The median percent lung lesion scores were 12.9 percent for the Fostera PRRS group and 25.4 percent for the non-vaccinated challenge control group (see Table 1).
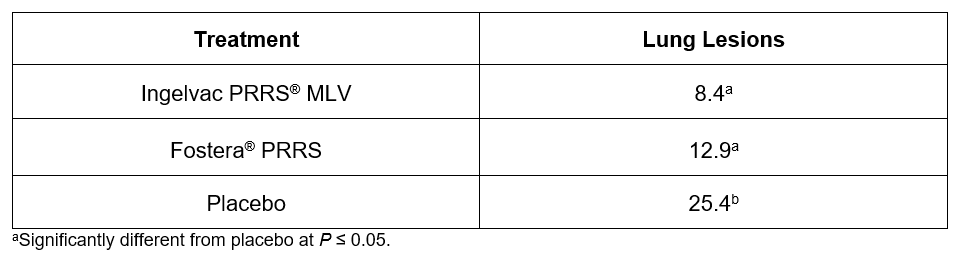
Table 2 summarises the post-challenge ADWG for all three groups. The Ingelvac PRRS MLV group had the highest ADWG at 0.61 pound per day. Fostera PRRS pigs gained an average of 0.49 pound per day, with 0.24 pounds for non-vaccinated challenge control pigs.
At D42 (14 days post challenge), levels of viremia were significantly lower in pigs vaccinated with Ingelvac PRRS MLV in comparison to pigs in the non-vaccinated challenge control group and Fostera PRRS group (P < 0.001 and P < 0.0017, respectively).
Results of these studies show that Ingelvac PRRS MLV provides cross-protection against a current and relevant PRRSv 1-7-4 challenge. Boehringer Ingelheim researchers have conducted multiple challenge studies against wild-type PRRSv strains since the introduction of Ingelvac PRRS MLV in 1994, and the vaccine has demonstrated cross-protection in every study.
| References | ||||
|---|---|---|---|---|
| 1 Patterson A, Victoria J, Jordan D, et al. | ||||
| (2013) | Modified-live PRRS vaccination is efficacious following challenge with eight genetically diverse PRRSv isolates, in Proceedings. | |||
| 2 Patterson A, Haiwick G, Hermann J, et al. | ||||
| (2017) | Efficacy of Ingelvac PRRS® MLV against a heterologous PRRSv 1-7-4 RFLP challenge. | |||







