



Comparative Evaluation of One- and Two-Dose PCV and M. hyopneumoniae Vaccination Protocols in Swine Challenged with PCV and M. hyopneumoniae
A dual-challenge study by Zoetis demonstrated that a one- or two-dose regimen of Fostera PCV effectively controlled PCVAD and reduced PCV2 viraemia and faecal shedding, which helped vaccinated pigs sustain favorable growth performance.Porcine circovirus-associated disease (PCVAD) caused by porcine circovirus Type 2 (PCV2) encompasses several distinct clinical syndromes that can include respiratory disease, enteritis, reproductive failure, porcine dermatitis and nephropathy syndrome, and systemic infection typified by unthriftiness and wasting. Viremia and protracted viral shedding are major features of PCVAD. In addition, subclinical infection is characterized by lymphocytic depletion in lymph nodes often accompanied by histiocytic infiltration. Given this wide scope of physiological involvement, it is not surprising that PCVAD adversely impacts production performance.
Higher levels of PCV2 viremia are associated with lymphoid depletion and clinical PCVAD, particularly when co-infections are present.1,2 Viremic pigs can be immunocompromised and more susceptible to other pathogens such as porcine reproductive and respiratory syndrome virus (PRRSV), Mycoplasma hyopneumoniae (M. hyo), and swine influenza virus (SIV).3 Viral shedding from multiple routes (fecal, oronasal, urinary, colostral) occurs rapidly after PCV2 exposure, enabling easy transmission to susceptible pigs.2 Thus, viremia and viral shedding are particularly relevant measures for evaluating the efficacy of PCV2 vaccines in challenge studies. The ability of a vaccine to help limit or avoid viremia is an important factor in helping minimize the negative economic consequences of disease.
Fostera™ PCV and RespiSure-ONE®
Fostera PCV, from Zoetis, is the only single-dose vaccine labelled to help prevent PCV2 viremia. Fostera PCV is licensed for vaccination of healthy pigs 3 weeks of age or older as an aid in preventing viremia and as an aid in the control of lymphoid depletion caused by PCV2. A 4-month duration of immunity has been demonstrated when administered as a single 2-mL dose. In addition, Fostera PCV is the only PCV vaccine that offers protocol flexibility, allowing administration as a single 2-mL intramuscular (IM) dose, or as two 1-mL IM doses given three weeks apart.
RespiSure-ONE is the single-dose M. hyo vaccine from Zoetis that allows producers to vaccinate for mycoplasmal pneumonia when they process baby pigs. RespiSure-ONE is the only mycoplasmal pneumonia vaccine that can be given as early as day 1, an important distinction because M. hyo may infect pigs within the first three weeks of life.4 RespiSure-ONE is also the only vaccine labelled to aid in reducing shedding and severity of colonization of and chronic pneumonia caused by M. hyo. Administered as a single 2-mL IM injection, RespiSure-ONE has demonstrated a 25-week duration of immunity for pigs vaccinated at three to eight days of age.
A study was conducted to compare vaccination regimens using Fostera PCV and RespiSure- ONE and two other competitive vaccines/ regimens in their ability to limit PCV2 viremia, reduce fecal shedding, and sustain favorable growth performance in swine challenged with both M. hyo and virulent PCV2b.5
Experiment Design
The study involved 412 PRRS and M. hyo negative baby piglets (barrows and gilts) that were farrowed at a high-health herd. At processing (three to seven days of age), piglets were ear-tagged, weighed, and randomly assigned to five treatment groups by blocks based on body weight and gender. The ‘vaccination phase’ of the study (zero to six weeks of age) involved administration of the following products:
- Circumvent® PCV M (2 doses), Merck;
- Ingelvac CircoFLEX-MycoFLEX® (1 dose mixed into a single bottle before vaccination), Boehringer Ingelheim Vetmedica;
- RespiSure-ONE (1 dose) and Fostera PCV (1 or 2 doses), Zoetis.
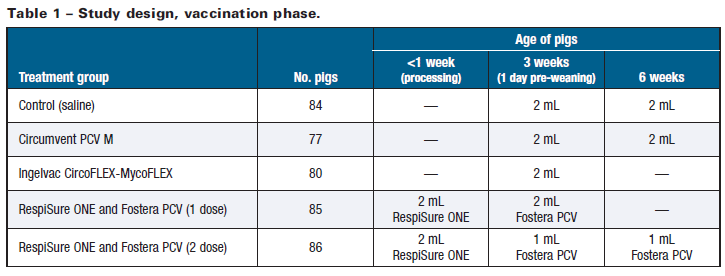
With the inclusion of a saline-injected control group, five treatment groups were thus evaluated as summarized in Table 1, with all IM vaccinations administered according to label directions.
Pigs were weaned at three weeks of age, moved to a wean-to-finish barn, and thereafter housed in pens by treatment group for a total of 15 pens.
The ‘challenge phase’ of the study (8-10 weeks of age) involved two separate events:
- M. hyo: at approximately 8 weeks of age (16 days after the vaccination phase), each pig was challenged intratracheally;
- PCV2b: at approximately 10 weeks of age, each pig was challenged via both the IM and intranasal (IN) routes.
Feed and water were offered ad libitum throughout the study, with feed formulated to contain an appropriate nutrient profile for optimal lean gain (six stages).
Pigs were observed daily for general health and clinical signs of respiratory distress, lethargy, wasting, etc. To allow computation of average daily gain (ADG), pigs were weighed at multiple time points throughout the study until marketing at approximately 260 lb (151 days of age, around 22 weeks). Pen feed consumption was also monitored to allow calculation of feed efficiency (feed/gain). Individual serum samples were collected at approximately 1, 3, 6, 8, 10, 14, 17 and 21 weeks of age and analyzed by polymerase chain reaction (PCR) for detection of PCV2 viremia as well as serological assessments and screenings (e.g., to confirm absence of PRRSV or SIV co-infections). Fecal swabs were collected for PCV2 shedding evaluations (using PCR) from 9 to 11 randomly selected animals in each treatment group at approximately 4, 7, and 11 weeks after PCV2 challenge (14, 17 and 21 weeks of age, respectively). The same pigs were used for each fecal shedding sample date. Removals, withdrawals, and deaths were documented for date, cause, and weight. Mortalities were necropsied and histological examinations performed to determine if death was due to PCV2 infection.
Data were statistically analyzed by appropriate methods using each pig as an experimental unit, with statistical significance recognized at P≤0.05. Viremia, fecal shedding, and ADG results were analyzed as least squares (LS) means. Clinical observations, data collections, and assays were conducted by personnel without knowledge of treatment group assignments.
Results
The virulence of the PCV2 challenge was evidenced by three deaths in the control group due to PCVAD (PCV-positive by PCR and IHC, plus lymphoid depletion and weight loss). Ten other mortalities occurred across the five treatment groups due to causes unrelated to PCV2 disease. No coinfections with PRRSV were detected, but 86 per cent of samples were positive for SIV by NP ELISA at study conclusion. The M. hyo challenge (serologically confirmed) appeared to be successfully moderated by M. hyo vaccination (based on reduction of lung lesions in vaccinates; data not shown).
The intensity of PCV2 viremia experienced by the various treatment groups is summarized in Figure 1. On each post-challenge sample day, all vaccinated groups demon strated viremia that was several orders of magnitude lower than the control group (P ≤ 0.05). These outcomes suggest that each PCV2 vaccine helped reduce viremia, and/or viremia was exacerbated in the control group due to infection with M. hyo. Both the 1-dose and 2-dose Fostera PCV groups demonstrated excellent viremia control all the way to market weight, confirming that a high and sustained degree of viremia protection was afforded by either dosing option.
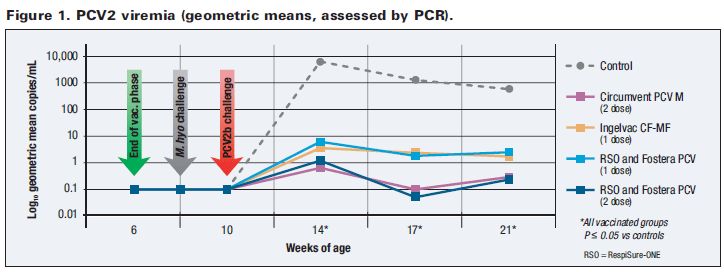
As a result of reduced viremia, fecal shedding of PCV2 was also reduced by vaccination (Figure 2). Incidence of fecal shedding fell moderately in all vaccinated groups relative to controls by 4 weeks post-challenge, with dramatic reductions detected by 7 weeks post-challenge. Notably, no sampled pigs vaccinated with the 2-dose Fostera PCV regimen were shedding PCV2 at 7 and 11 weeks after challenge. These results suggest that Fostera PCV (the 2-dose regimen, in particular) can help limit the quantity of PCV2 shed, thereby helping reduce PCV2 exposure for other pigs.
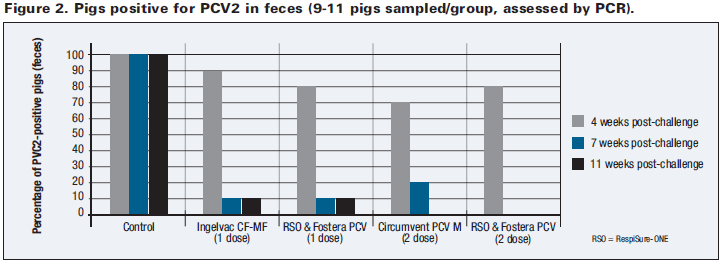
ADG outcomes for the study are summarized in Table 2. During the immediate 9 weeks following the vaccination phase of the study (6-15 weeks of age, encompassing 5 weeks of post-challenge performance), all vaccinated groups generated sizeable ADG improvements (6.2 per cent-8.5 per cent) relative to controls (P ≤ 0.05). No differences in ADG were detected between vaccinated groups for this time period (P > 0.05).
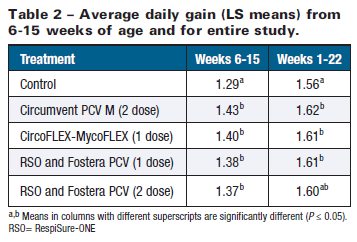
Overall ADG results (piglet processing to market, weeks 1-22) also revealed significant (P≤0.05) improvement for vaccinates compared to controls (about 3.5 per cent), with the exception of the two-dose Fostera PCV group (P=0.1084). Again, growth performance outcomes between vaccinated groups were similar (P>0.05). No differences in feed efficiency were detected between any treatment groups over the course of the study (P>0.05; data not shown).
Though the favorable impacts of reduced PCV2 viremia, reduced PCV2 fecal shedding, and M. hyo control on ADG were substantial, performance benefits may have been moderated by the low level of co-infections, as dictated by trial design. Under more typical production conditions where exposure to PCV2 and other pathogens coincide, the favorable impacts of reduced PCV2 viremia and fecal shedding on herd performance could likely be amplified.
Conclusions
This dual-challenge study demonstrated that a one- or two-dose regimen of Fostera PCV effectively controlled PCVAD and reduced PCV2 viremia and fecal shedding, which helped vaccinated pigs sustain favorable growth performance. No differences (P>0.05) in viremia, shedding, or ADG outcomes were detected between the Fostera PCV regimens and competitor vaccines/ regimens. In addition, the M. hyo challenge infection was successfully controlled by early vaccination with RespiSure-ONE.
Fostera PCV represents an effective and versatile vaccine that helps provide excellent control of PCV2 viremia, which is a critical feature for helping reduce fecal shedding (exposure risk for other pigs) and for sustaining profitable growth performance.
References
1. Allan GM, Ellis JA. Porcine circoviruses: a review. J Vet Diagn Invest 2000; 12:3-14.
2. Opriessnig T, Meng XJ, Halbur PG. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest 2007; 19:591-615.
3. Harms PA, Sorden SD, Halbur PG, et al. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet Pathol 2001; 38:528-539.
4. Fano E, Pijoan C, Dee S, Deen J. Effect of Mycoplasma hyopneumoniae colonization at weaning on disease severity in growing pigs. Can J Vet Res 2007; 71:195-200.
5. Data on file, Study Report No. 12ORBIOPORK01, Zoetis Inc.
Acknowledgement:
Deborah M. Amodie, MS, Biometrician
Further Reading
Find out more information on the diseases mentioned by clicking here.
Visit the PMWS/PCVD page by clicking here.
August 2013







