



Effects of DRAXXIN at Weaning for Control of Swine Respiratory Disease in Pigs Experiencing a Natural Outbreak
DRAXXIN, as an injectable product that provides an extended therapeutic period for the treatment of respiratory disease, was associated with the improved post-weaning performance of pigs in a flow with a high level of PRRS virus involvement and a mix of bacterial agents, several of which are well-established contributors to swine respiratory disease (SRD), according to a study by James W. Eubank, DVM and Robyn Fleck, DVM of Zoetis Inc.Key Points
- A study was conducted under field use conditions to determine whether weaned pigs administered a single injection of DRAXXIN® (tulathromycin) upon entering the nursery for the control of bacterial infections associated with swine respiratory disease (SRD) would show production advantages.
- Evaluated in the study were: wean-to-finish percent mortality, percent pulls to the hospital pen, percent pigs re-treated, nursery weight gain, and wean-to-finish weight gain.
- Pigs that had received DRAXXIN in comparison to pigs that received a placebo injection in the control group:
- had a significantly (P≤0.05) lower mortality (16.83 per cent versus 21.4 per cent), and,
- as a derived benefit of a reduction in SRD, gained 4.2 lb more on average by the first marketing, a significant (P≤0.05) difference.
- Pigs treated with DRAXXIN had significantly (P≤0.05) fewer gilts requiring antibiotic re-treatments (40.5 per cent versus 56.9 per cent).
- Pigs treated with DRAXXIN had significantly (P≤0.05) fewer overall pulls to a hospital pen (3.04 per cent versus 7.18 per cent).
- DRAXXIN for control of SRD provided a 15:1 return on investment:
- The DRAXXIN-treated group of 550 pigs produced 8,137 more pounds of pork than the 550 control pigs.
- The DRAXXIN-treated group marketed 29 more pigs than the control group.
- Comparative weight distribution results showed that more DRAXXIN group pigs reached market weights sooner than control group pigs as a derived benefit resulting from the control of SRD, which suggests that even greater return on investment might be realised as more pigs would hit packer premium price targets, depending on when they leave the finisher.
DRAXXIN (tulathromycin) Injectable Solution has been formulated for intramuscular (IM) injection as a ready-to-use single dose (2.5 mg/kg). DRAXXIN is indicated for the control of SRD associated with Actinbacillus pleuropneumoniae, Pasteurella multocida and Mycoplasma hyopneumoniae (M. hyo). DRAXXIN also provides a full course of treatment against key bacterial pathogens (A. pleuropneumoniae, P. multocida, Bordetella bronchiseptica, Haemophilus parasuis, and M. hyo1) associated with SRD.2,3,4,5 DRAXXIN persists in the lung tissues, providing a nine-day window of coverage.6 The pre-slaughter withdrawal time for DRAXXIN Injectable Solution in swine is five days. DRAXXIN should not be used in animals known to be hypersensitive to the product.
The study summarised below was conducted using commercially produced weaned pigs over a 152-day period to evaluate the effects of administering a single intramuscular dose (2.5mg per kg body weight) of DRAXXIN for the control of SRD at weaning. Percent mortality, percent pulls to a hospital pen, percent pigs requiring antibiotic re-treatments, nursery weight gain, and wean-to-finish weight gain were determined in DRAXXIN-treated pigs in comparison with saline placebo-treated pigs.
Study Overview: Design, Assessments and Analysis
Study design
- The study was conducted in a contract research organisation in Nebraska using locally sourced commercial weaned pigs from PRRS-positive, M. hyo-positive sources. At arrival, 17 pigs were euthanised and necropsied and SRD was confirmed by a licensed veterinarian.
- 1,100 head of pigs were sourced from two commercial sow farms and placed into an all-in/all-out (AIAO) nursery room.
- On the day of arrival to the nursery, the newly weaned pigs were uniquely identified with ear tags and stocked in pens to ensure the same square foot per pig.
- The pigs were maintained on un-medicated nursery starter feed for the first 21 days of the trial. They did not receive any water medications during this time.
- After the nursery phase, the pigs were moved to an AIAO finisher barn until market.
Day 0
- Using a separate randomisation schedule for gilts and barrows and for each sow farm source, pigs were individually weighed and enrolled into either DRAXXIN treatment or saline controls using a blocked design. The pigs from each treatment remained commingled in their pens (Table 1).
- Saline treated control pigs (n=550) were administered 1.0mL sterile saline per 88-lb body weight IM.
- DRAXXIN treated test pigs (n=550) were administered 1.0mL DRAXXIN per 88-lb body weight IM.

- Blood samples were collected from 60 uniquely tagged subset pigs.
- Serial blood samples were collected from these pigs at days 0, 33 and 98.
- PRRSV PCR assays were run on pools of five at days 0 and 33.
- Ten of the 60 subset pigs were left unvaccinated for M.hyo and M.hyo titres were tested via ELISA on days 0, 33 and 98.
Day 54
- Pigs were weighed a second time.
Day 152
- Pigs were weighed a final time before the first animals were marketed.
Days 0-152
- Each pig that died was necropsied by a veterinarian and the cause of death determined.
- Bacterial cultures were performed on the lungs of the first 73 pigs that died or were euthanised and diagnosed with SRD.
- Any pig that had a respiratory or depression score of 1 or above (scoring system ranged from 0–3) was re-treated with EXCEDE® for the first re-treatment or Baytril® for the second and third re-treatments. A seven-day treatment moratorium was observed between treatments to enable the pig to respond.
- Any pig that was deemed unable to compete in its home pen was pulled to a hospital pen.
Statistical analysis
- The individual animal was the experimental unit for all statistical analyses.
- Initial body weight, weight gain and average daily gain (ADG) were analysed by a Linear Mixed Model (LMM) approach.
- Percent re-treatments, hospital pen moves and mortalities were defined as binary variables (yes=1, no=0) and analyzed using a Generalized Linear Mixed Model (GLMM).
- If the interaction of treatment-by-gender was significant at the five per cent level for any parameter, an analysis was performed for that parameter separately for each gender.
Results
SRD outbreak
- Pigs were PRRS-positive at arrival in that 8/12 pools of serum were PCR-positive to PRRS. The lowest cycle time was 30.32 for one pool, indicating a heavy infection. By day 33, 1/11 pools was positive and the lowest cycle time was 31.4, indicating persisting PRRS viraemia.
- PRRS outbreaks such as this one in newly weaned pigs can produce significant mortality due to secondary bacterial infections.7,8,9
- Necropsies on all animals indicated that there was a large component of SRD, with the saline treated pigs having 89 deaths due to SRD, compared with the DRAXXIN pigs, which had 48 deaths (Table 2).
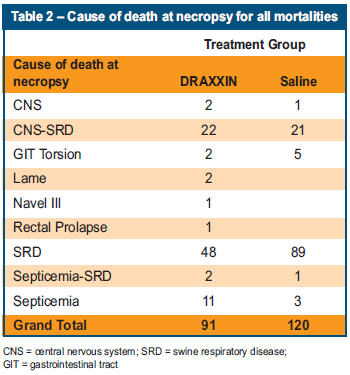
- Besides SRD, Streptococcus suis meningitis, which is commonly associated with PRRS outbreaks,10,11 was found in both groups (Table 2).
- 5/11 of the M.hyo unvaccinated sentinel pigs were positive to M.hyo at weaning, indicating presence of maternal antibodies. By study day 21, 0/10 were positive, but by study day 98, 5/6 pigs were positive, indicating that M.hyo exposure had occurred during the growing period.12,13
Percent Death Loss
- Pigs in the study group treated with DRAXXIN at weaning had 29 more live pigs at first marketing than the saline treated group (P<0.05). The difference in mortality was a result of controlling SRD in the DRAXXIN treated pigs (Table 3).
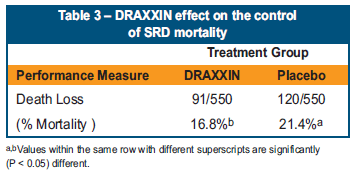
- DRAXXIN treated pigs were 24 per cent less likely to die than saline treated pigs.
- For every 19 pigs treated with DRAXXIN at weaning, one pig was prevented from dying.
Nursery and finisher weight gain
- Pigs in the study group treated with DRAXXIN at weaning gained 42.86 lb by day 54 versus 40.65 lb in the saline treated control pigs, for a difference of 2.21 lb at the end of the nursery phase (P<0.05) (Table 4).
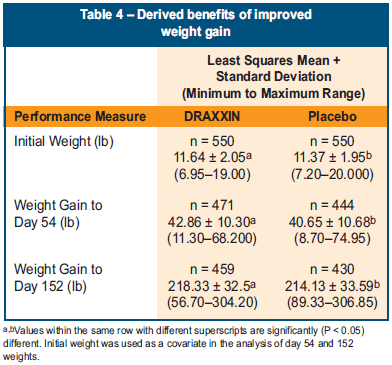
- Pigs in the DRAAXIN-treated group gained 218.33 lb from weaning to first marketing versus the saline treated control pigs that gained 214.13 lb. This difference of 4.2 lb of weight gain was statistically significant (P<0.05) (Table 4).
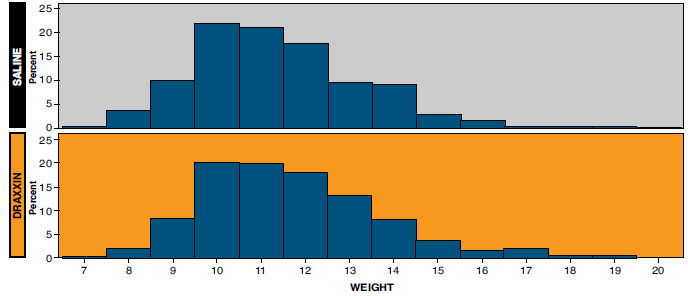
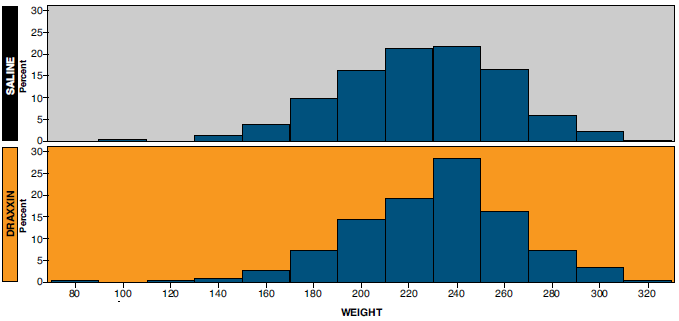
- Comparison of the distribution of weights at day 0 (Figure 1) and at day 152 (Figure 2) demonstrates that the population of smaller pigs on the left side of the distribution was reduced in the DRAXXIN-treated pigs by the time of first marketing. This made more pigs available to the packer grid in the DRAXXIN-treated group than the saline treated group.
Re-treatments and pulls to hospital pen
- Gilts given DRAXXIN at weaning were 31 per cent less likely to require re-treatment with another antibiotic versus those receiving saline (P<0.05). There were also numerically fewer barrows requiring re-treatments in the DRAXXIN treated animals than those receiving saline (Table 5).
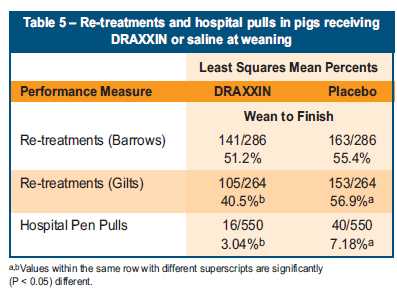
- Pigs receiving DRAXXIN at weaning were 60 per cent less likely to be pulled to a hospital pen than those receiving saline (P<0.05) (Table 5).
Economic analysis
Performance measurements for each phase of the study are presented in Table 6. The economic analysis was based upon the difference in the pounds of pork produced up to day 152. The cost of DRAXXIN ($421.95, based on the 100-mL bottle price) represented the sole input variability. The effects associated with taking additional inputs into account are expected to provide even greater benefits.
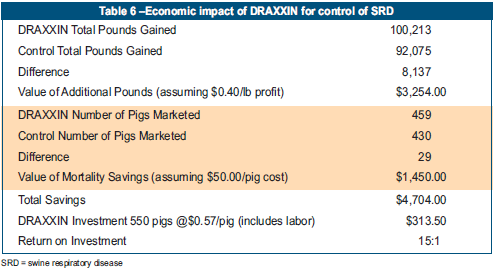
- Because SRD mortality was controlled in the nursery, pigs treated with DRAXXIN produced 8,137 more pounds of pork at the end of the finisher phase as compared with the same number of pigs treated with saline.
- Total value of the extra pounds of pork was estimated at $3,254.80 based on a profit (additional revenue minus additional feed cost) for the pork of $0.40 per lb.
- Based on an estimated value of $50.00 for each dead pig (weaned pig purchase price, feed, vaccines, transportation etc), it was determined that the 29 extra head that survived in the DRAXXIN group provided a comparative savings of $1,450.00 versus saline controls.
- The total value to DRAXXIN was realised through prevention of losses ($1,450.00) plus additional profit from weight gain ($3,254.80), which totaled $4,704.00.
- Assuming an input cost of ($313.00) to treat 550 pigs, investigators determined that the return on investment for controlling SRD in weaned piglets was 15:1.
Conclusion and Discussion
The study showed that administration of DRAXXIN at weaning reduced mortality in pigs in a flow harboring multiple bacterial and viral agents associated with SRD. The resulting improvement in pig performance resulted in increased economic returns. The distribution of weight among DRAXXIN treated pigs suggests that even greater returns on investment might be realized as more treated pigs would hit packer premium price targets upon leaving the finisher. Similar health and performance improvements were reported previously in a trial in which DRAXXIN was administered to weaned pigs contact exposed to multiple bacterial and viral pathogens – including PRRSV – associated with the SRD.14
Whereas the potent antibacterial and anti-mycoplasmal activities of DRAXXIN are well documented and likely have a positive effect on production, no studies have been conducted to identify whether ancillary benefits might result from the administration of this novel antimicrobial.
The study reported here illustrated that DRAXXIN, as an injectable product that provides an extended therapeutic period for the treatment of respiratory disease, was associated with the improved post-weaning performance of pigs in a flow with a high level of PRRS virus involvement and a mix of bacterial agents, several of which are well-established contributors to SRD.
Important Safety Information: The pre-slaughter withdrawal time for DRAXXIN Injectable Solution in swine is five days. DRAXXIN should not be used in animals known to be hypersensitive to the product. Please see full prescribing information.
References
1. McKelvie J., Morgan J.H., Nanjiani I.A., Sherington J., Rowan T.G. and Sunderland S.J. 2005. Evaluation of tulathromycin for the treatment of pneumonia following experimental infection of swine with Mycoplasma hyopneumoniae. Vet Ther 2005;6(2):197–202.
2. Data on file, Study Report No. 1123R-60-07-283, Zoetis Inc.
3. Hoover T.C. Health and performance improvements of pigs treated with DRAXXIN (tulathromycin) injectable solution for swine respiratory disease under field conditions. Pfizer Animal Technical Update June 2011:1–4. Publication DXS11010.
4. Nutsch R.G., Hart F.J., Rooney K.A. et al. Efficacy of tulathromycin injectable solution for the treatment of naturally occurring swine respiratory disease. Vet Ther 2005;6:214–224.
5. Nanjiani I.A., McKelvie J., Benchaoui H.A., et al. Evaluation of the therapeutic activity of tulathromycin against swine respiratory disease on farms in Europe. Vet Ther 2005;6(2):203–213.
6. Benchaoui H.A., Nowakowski M., Sherington J. et al. Pharmacokinetics and lung tissue concentrations of tulathromycin in swine. J Vet Pharmacol Therap 2004;27:203–210
7. White M. Porcine respiratory disease complex (PRDC) Part 1: Introduction. Livestock 2011;16(2):40–42.
8. Brockmeier S.L., Halbur P.G, Thacker E.L. Porcine respiratory disease complex. In: Brogden KA, Guthmiller JM (eds). Polymicrobial Diseases. Washington DC: ASM Press;2002:231–258.
9. Stevenson G.W., Van Alstine W.G., Kanitz C.L. Characterization of infection with endemic porcine reproductive and respiratory syndrome virus in a swine herd. JAVMA 1994;204(12):1938-1942.
10. Feng W-H., Laster S.M., Tompkins M. et al. In utero infection by porcine reproductive and respiratory syndrome virus is sufficient to increase susceptibility of piglets to challenge by Streptococcus suis Type II. J Virol 2001;75(10):4889–4895.
11. Galina L., Pijoan C., Sitjar M. et al. Interaction between Streptococcus suis serotype 2 and porcine reproductive and respiratory syndrome virus in specific pathogen-free piglets. Vet Rec 1994;134(3):60–64.
12. Thacker E.L., Halbur P.G., Ross R.F. et al. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J Clin Microbiol 1999;37:620–627
13. Halbur P.G. “PRRS Plus”—PRRS virus infection in combination with other agents. In: Fact Sheet Pork Information Gateway, PRRS Compendium Producer Edition 2009;11–16.
14. King D., Fleck R., Amodie D. Effects of Draxxin at Weaning in pigs on control of swine respiratory disease including “low” level of PRRS involvement and subsequent performance of pigs. Proceedings of the American Association of Swine Veterinarians, 2013 San Diego, CA, poster 97.
Further Reading
Find out more information on the diseases mentioned in this article by clicking here.
July 2013







