



Understanding the Generation and Chemistry of Odours
Wendy Powers of Iowa State University offers an introduction to the chemistry of odours, how they are classified and why they may become a nuisance from farms.Introduction
Odour chemistry is complex and still poorly understood. More than 75 odorous compounds, in varying proportions, have been identified in livestock manures. Knowing the chemical basis of odours derived from animal manure is helpful to understand how odor develops and what can be done to design and manage manure systems and avoid nuisance complaints.
Objectives
The objectives of this factsheet are to describe:
- the formation and primary groups of odorous compounds in manure
- the impact of the physical environment on odour release from manure.
- methods for characterisuzing odour.
- the impact of odour interactions.
Biochemistry of Manure Odour
Groups of primary odorous compounds include volatile organic acids, aldehydes, ketones, amines, sulphides, thiols, indoles and phenols. All of these groups can result from the partial decomposition of manure. Manure break-down is accomplished by a mixed population of anaerobic bacteria, which is commonly grouped into acid-forming or methane-producing classes. Acid formers are responsible for the initial break down of complex molecules into short-chain compounds,
including organic acids. Methane bacteria further reduce organic acids to methane and carbon dioxide.
Figure 1 provides a simple overview of the breakdown process. The break-down of protein proceeds to ever-simpler proteoses, peptones, peptides, amino acids and finally, to ammonia and volatile organic acids such as formic, acetic, propionic and butyric acids. Due to the presence of sulphur in certain amino acids - sulphur averages about one per cent of most proteins - various sulphides and mercaptans can be expected as a result of protein catabolism.
Carbohydrates in animal waste include sugars, starch and cellulose. Starch and cellulose are broken into glucose (sugar) units as the first step of decomposition. Under anaerobic conditions, sugars are broken into alcohols, aldehydes, ketones and organic acids. These intermediate compounds are odorous and can be further metabolised and transformed into methane, carbon dioxide and water (non-odorous end-products) if conditions allow the methane-producing microorganisms to function.
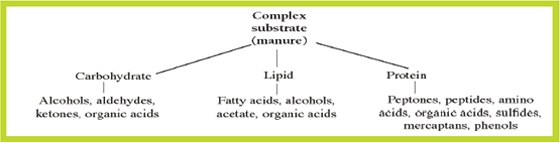
Fats are esters of the tri-hydroxy alcohol called glycerol. Bacteria use fats as an energy source, hydrolysing them first to the corresponding long-chain fatty acids and alcohols. These acids, along with those produced in the deamination of amino acids, undergo further breakdown in which acetic acid is cleaved from the original acid. Acetic acid is then potentially utilised as an energy source, yielding methane and carbon dioxide as end-products.
Examination of the metabolic pathways for the breakdown of manure components indicate that the components are expected to result in: organic acids, alcohols, aldehydes, sulphides, simple hydrocarbons, carbon dioxide, ammonia and methane. The presence of this mixture of organic materials and ammonia in an aqueous solution leads to the formation of several other groups, as reaction products. For example, ammonia in water - an H+ receptor - may be expected to react with acids and alcohols to yield amides and amines. Also, hydrogen sulphide in water may combine with alcohols, aldehydes and acids to form mercaptans, thiols and thioacids.
An accumulation of these intermediate metabolites results in an offensive smelling product, whereas containment of intermediate compounds for sufficient time allows methane producers to act and metabolise most of the odorous compounds into non-odorous methane. Background levels of sulfur in water may also be a source of odour.
Physical Chemistry
Any compound occurring in the atmosphere must have escaped the liquid phase. Thus, vapour pressure is an important factor which, within specific types of compounds, generally decreases with increasing molecular weight.
The solubility of a compound in water is another important factor in evaluating its significance as an atmospheric constituent. Insoluble gases, such as methane, escape immediately after being produced, whereas more soluble compounds, such as ammonia, are retained in solution and can engage in biological and chemical reactions. Solubility of many compounds, and hence odor, is markedly influenced by the solution pH. Hydrogen sulphide is a particularly good example of the pH effect. Under conditions of high pH, almost no odour is detected whereas under acid conditions, the H+ and HS- ions combine, escape and produce the typical sulphide odour (H2S). Ammonia (NH3) is another good example of pH effect. The NH3 in an acid medium accepts H+ to produce ammonium (NH4+) which stays in solution and does not volatilise. Even with a pH up to 8, ammonia remains relatively soluble in liquids and little odour is detected. Above a pH of 9, however, ammonia is rapidly volatilised.
No single compound has been identified as a good predictor of odour sensation across situations in the field. Because of this, human panelists conduct odour measurements and quantify odour intensity and unpleasantness.
| Table 1. Concentrations of the seven primary odour classes required to produce equal odour intensity. [2] | ||
|---|---|---|
| Odour | Compound | Concentration (ppm) |
| Ethereal | Ethylene dichlor | 800 |
| Camphoraceous | 1,8 cineole | 10 |
| Musky | Pentadecanlacton | 1 |
| Floral | Phenylethylmethyl ethylcarbinol | 300 |
| Minty | Methone | 6 |
| Pungent | Formic acid | 50,000 |
| Putrid | Dimethyl disulphide | 0.1 |
Odour Characterisation
Based on psychological tests, seven primary classes of olfactory stimulants have been found to preferentially excite separate olfactory cells. These classes are: 1) ethereal, 2) camphoraceous, 3) musky, 4) floral, 5) minty, 6) pungent and 7) putrid. The nervous system integrates the responses from a number of cells to determine the identity of the primary odour stimulus being received.
The intensity of the perceived odour class is related to the number of receptors bound and the degree of excitation of the olfactory cells. Table 1 shows the variation in concentration needed to produce equivalent odour intensities in the seven classes. Odour intensity, as referred to in Table 1, is the strength of the odor sensation as measured on a psychological reaction scale and is not a concentration. Complex odours result from the concurrent stimulation of two or more types of receptors. This implies that a single chemical can occupy more than one receptor site.
The use of the seven primary odour classes is widely cited. However, it is unlikely that this list actually represents the true primary sensations of smell. More than 50 single substances have been identified in odour blindness studies, suggesting that there may be 50 or more sensations of smell.
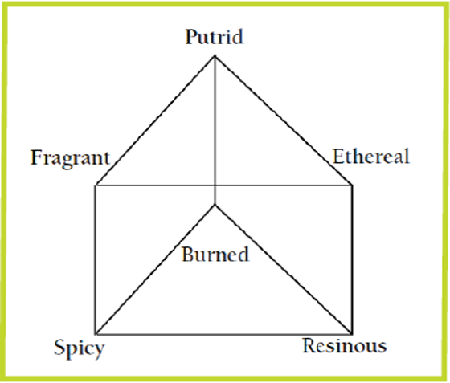
A more flexible way of presenting the primary odours to clarify the idea of complex odours is through the use of Henning's odour prism (Figure 2). Six primary odours are located at the corners of the prism. All other odours are mixtures of the primary odours and located on the surfaces and edges of the prism. Thus, odours consisting of two components would be represented on the edges of the prism, three-component mixtures occupy the triangular surfaces, and four-component mixtures occupy the square surfaces.
Odour Interactions
Usually, an odorous stimulus is a combination of many scents. This is certainly the case in animal production facilities. The effect of one odour on another may be related to differences in the water solubility of the two odours resulting in a number of possible outcomes. Flowery, fruity odorants tend to have higher molecular weights. Aldehydes, esters, alcohols, ethers,
halogens, phenols and ketones have more pleasant aromas than the lower molecular-weight carboxylic acids, nitrogenous compounds (not associated with oxygen) and sulphur-containing compounds. Blending of the two odours may occur, producing an odour with properties of both the original and properties unique to the newly-developed odours.
One odour may dominate another, or at least periodically, or the two odours may be smelled concurrently as individual odours. The complex nature of how odorants interact with each other is the primary challenge in determining how best to prevent odour formation. However, understanding that manure odours form as the result of incomplete breakdown of excreted products, and that many of these products are the result of excess protein in the diet can serve as the basis for odour management.
Summary
The primary odorous compounds result from the intermediate products in the anaerobic breakdown of carbohydrates, lipids and proteins in the manure. The rate of odour release from the liquid to gas phases is influenced by compound vapour pressure, pH and solubility. Odours can be classified using six primary classes and combinations thereof using Henning's odour prism. However, interactions between odorous compounds can result in an odour with properties of both the original and properties unique to the newly-developed odours.
References cited and other resources
[1] Powers, W. 2004. The science of smell Part 2: Odor chemistry. Iowa State University Extension Publication PM 1963B. Accessed 4-25-2011 .
[2] Water Environment Federation. 1978. Odor Control for Wastewater Facilities. Manual of Practice No. 22. Water Pollution Control Federation, Washington D.C.
[3] Powers-Schilling, W.J. 1995. Olfaction: chemical and psychological considerations.
Proc. of Nuisance Concerns in Animal Management: Odor and Flies Conference, Gainesville, Florida, March 21-22.
This paper is adapted from 'The Science of Smell Part 1: Odor perception and physiological response' by Wendy Powers, Iowa State University Extension Publication PM 1963A, May 2004 and can be found on the Air Quality and Animal Agriculture Web page.
Go to our previous article on the science of odours by this author by clicking here.
February 2013






