



Why We Should Reduce Antibiotic Usage and Ways to Do It
A thorough review by Robert Derosiers of Boehringer Ingelheim Canada, presented at the London Swine Conference 2013, including evidence of a whole range of interventions that could be used by pig producers to reduce the threat of antibiotic resistance in human and porcine pathogens.
Abstract
Antibiotics are vital for the health and wellbeing of both animals and man. The development of resistance to antibiotics is a major concern, and for that reason their use is under increased scrutiny. As is the case in the US and Mexico, the quantity of antibiotics used to raise pigs in Canada is significantly greater than in Denmark, where efforts to reduce their usage have been made for many years.
This paper discusses reasons why we should try to reduce antibiotic usage in Canada, and ways that can be used to obtain that result. Strategies abroad found to be successful on a country basis will be summarised, as well as options that individual producers could consider in their own herd.
Introduction
Since the discovery of penicillin by Sir Alexander Fleming in the 1920s, antibiotics have played an enormous role in Man’s quest for a better and longer life. Today, these products are still extremely important for the well-being of both humans and animals, and for that reason everything that can alter their efficacy is closely scrutinised. Antibiotics are used in swine production with various objectives in mind.
This paper will briefly touch on reasons why we should try to limit their usage whenever possible, and on how we can do it.
Why We Should Try to Reduce Antibiotic Usage
For some time now, the use of antibiotics in animals, particularly food-producing animals, has been a hot topic.
The main concern behind these discussions is that if antibiotics are used a lot in animals, veterinary pathogens or commensals may become more resistant to antimicrobials, and if so, could transfer that resistance to human pathogens. Furthermore, some organisms carried by pigs have the potential to create problems in humans, and these organisms may be directly transferred to people though manipulation or consumption of meat products. If these strains of organisms are resistant to antibiotics, the treatment of these conditions obviously becomes an issue.
The extent to which resistance in human pathogens could be associated with antibiotic usage in animals remains an open question, and the same is true for the impact that restricting access to antibiotics could have on animal health, economic performance and welfare. This being said, there seems to be little doubt that pressure to reduce antibiotic usage in animals will grow, and we certainly have had examples of that pressure recently in North America.
In March 2012, a poll involving 1,000 US residents revealed that 72 per cent of consumers were extremely or very concerned about overuse of antibiotics in animal feed, and 60 per cent were ready to pay five cents or more per lb for meat of animals raised without antibiotics (Moreno, 2012).
On 6 April 2012, the US Food and Drug Administration’s ban prohibiting the use of cephalosporins at unapproved doses, frequencies, duration or routes of administration, and perhaps more importantly, for disease prevention, became effective (US Food and Drug Administration, 2012).
In June 2012, an editorial in the Canadian Medical Association Journal was titled ‘Farm-grown superbugs: While the world acts, Canada dawdles’ (Sibbald, 2012). The editorial asked for stricter regulations on antibiotic use in animals in Canada, particularly for classes of antibiotics which are of primary importance in human medicine. But this issue of antibiotic usage and resistance is indeed a global one.
In a speech given last year at a conference on antimicrobial resistance in Copenhagen, Dr Margaret Chan, director-general of the World Health Organisation, stated: “The antimicrobial threat is easy to describe. It has an irrefutable logic. Antimicrobial resistance is on the rise in Europe, and elsewhere in the world. We are losing our first-line antimicrobials. Replacement treatments are more costly, more toxic, need much longer durations of treatment, and may require treatment in intensive care units. For patients infected with some drug-resistant pathogens, mortality has been shown to increase by around 50 per cent.” (Chan, 2012).
All this suggests that the pressure from the human medical side for tighter controls and reduced usage of antibiotics in animals will increase. This is particularly true when efforts made elsewhere have proven that it was possible to raise animals, including pigs, with less antibiotics.
But the concerns over the potential impact that antibiotic usage in animals may have on human pathogens is only one aspect to consider. Animals also get sick and have to be treated, and for that effective antibiotics are needed.
Since it is believed that the introduction of new antibiotic molecules for use in animals is likely to be very limited in the future, we need to make sure that those we have today remain effective on a long term basis. For example, in Quebec, resistance of porcine Escherichia coli isolates to ceftiofur, one of our last resort drugs, went from close to zero in 1994 to more than 20 per cent in 2011 (MAPAQ, 2012). In Italy, Luppi et al. (2012) reported that none of the E. coli strains isolated from swine and tested in 2002 was resistant to more than 10 antibiotics, while it gradually increased to 25.8 per cent of the strains in 2011. In a recent study conducted in Ontario (Park, 2013), 97 per cent of the Staphylococcus hyicus (the cause of exudative epidermitis, or greasy pig disease) isolates, were resistant to penicillin G and ampicillin, and 71 per cent to ceftiofur.
Not all data sets on antibiotic resistance in swine pathogens are showing that kind of negative progression or picture, but we should all be concerned about the potential danger it represents.
Where do Canada and North America Stand?
Before we get into ways that can be used to reduce antibiotic usage, it appears logical to look at where we, in North America, stand. In other words, are we using less, the same or more antibiotics in Canada, the US and Mexico than countries like Denmark for example, where special efforts towards antibiotic usage reduction have been made for many years?
At the 2010 International Pig Veterinary Society meeting in Vancouver, Danish authors reported that between 2004 and 2009, the total quantity of antibiotics used in pigs in their country varied between 3.54 to 4.03g per pig (Stege, 2010).
Because no such data were seemingly available in North America, in the summer of 2011, the author tried to compile numbers on antibiotic usage in a few herds or companies in Canada, the US and Mexico that were representative of the North American industry.
Since this evaluation involved a very small number of farms and had no scientific pretentions at all, the numbers will not be mentioned here, but they suggested that while not worse than in the US and Mexico, the situation in Canada can be improved.
Of course, the total quantity of antibiotic used in pigs and other animals is only one parameter to evaluate. The type of antibiotic used is another very important one. As we will see later, chlortetracycline does not have the same relative importance as fluoroquinolones or third and fourth generation cephalosporins. Nevertheless, an important point can already be made at this stage: we need to know how much of the various antibiotics we are using in pig production in Canada. We need scientifically sound data that will allow us to accurately know where we currently stand. With this in hand, not only can we compare ourselves with other countries but we can also determine if the actions we will eventually take are producing results or not.
Putting that aside, let us assume that we can do better, and the rest of this document will look at some of the ways that can be used to reduce antibiotic usage in swine production.
What Have Other Countries Done?
A logical approach seems to be looking at what other countries have already done to reduce antibiotic usage in animals, and we will use Denmark and the Netherlands as examples.
Efforts to reduce antibiotic usage in animals in Denmark have been the topic of many articles and discussions, some being very positive, others suggesting that their strategy has actually not improved their consumption much over the years. Where does truth actually lie? Figure 1 shows the antimicrobial consumption in Danish pork production from 1992 to 2008. Antimicrobial consumption is defined as the number of milligrams of active compound per kilogram of pig produced (Aarestrup, 2010).
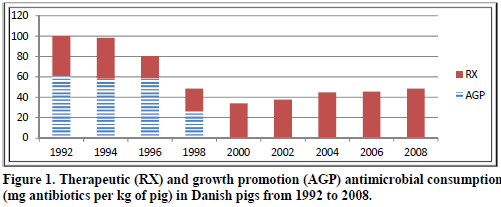
As can be seen, more antibiotics were used for growth promotion than for therapeutic use in 1992. But then various measures were taken to phase out growth promotion use of antimicrobials and in 2000, antimicrobials were not used anymore for that purpose. This did create an increase in the therapeutic use of these products, but the end result is still that by 2008, Danish pig production was using less than 50 per cent of the total they were using in 1992.
An international review panel, set up by WHO at the request of the Danish government, concluded that the ban reduced human health risks without significantly harming animal health or farmers’ incomes. In fact, Danish government and industry data showed that livestock and poultry production actually increased following the ban, while antibiotic resistance on farms and in meat declined (Chan, 2012).
Nevertheless, as the antimicrobial consumption was steadily increasing after 2000, they decided in 2010 to put in place some additional measures to stop and hopefully reverse that trend. Since then, if a producer uses two times or more the average quantity of antimicrobials used by Danish producers, he/she receives a yellow card. The producer then has nine months, working with his or her herd veterinarian, to correct the situation. If this does not work, another veterinarian gets involved in the farm, and if this still does not produce the desired results after five months, other measures are discussed but not currently implemented. One of the potential measures would be a decrease of animal inventory until antimicrobial consumption goals are met.
The yellow card system was initiated in July 2010, and the Danish figures are showing an impressive decrease of 19 per cent in pig antimicrobial consumption in 2011 (News, 2012). While this may not be totally due to the new system, the results are very encouraging. Furthermore, Alban et al. (2012) reported that this substantial decrease was obtained without animal health and welfare being deteriorated.
In 2009, a plan was instituted in the Netherlands to reduce antibiotic usage in animals by 20 per cent in 2011, and by 50 per cent in 2013. If the objectives were not reached, one of the measures considered was for veterinarians to lose the right to sell drugs. Veterinary prescriptions are mandatorily declared to public authorities through an information system called VetCis. The emphasis has been placed on biosecurity, nutritional strategies and vaccination, and between 2009 and 2011, the total sales of antibiotics decreased by nearly 32 per cent overall for the five livestock sectors considered (Bondt, 2012). Specifically on the swine side, the number of daily dosages per sow and piglets (a different way to calculate antibiotic consumption) went from 25 daily dosages per year in 2009, to 13 in 2011, and from 16 to 8 daily dosages per year in finishing pigs.
On a country basis, there are thus collective measures, guidelines or laws that have been used successfully to reduce antimicrobial usage in animals. But what about what producers and veterinarians can do in individual farms? Many different ways and alternatives can be considered, and the rest of this paper will briefly describe some of them.
Ways to Reduce Antibiotic Usage in Swine Production
Health improvement and maintenance
The most effective way to reduce antibiotic usage in pigs is to improve their health status, and maintain it at that improved level. Of course, this is not always easy, but the example given for this particular point is, in the author's opinion, quite impressive.
Five different pathogens (toxigenic Pasteurella multocida, Sarcoptes scabiei, Mycoplasma hyopneumoniae, Actinobacillus pleuropneumoniae and PRRS virus) were eliminated from a small purebred herd of 100 sows selling replacement gilts and boars (Desrosiers, 2001). Dr Réal Boutin implemented a programme based on early weaning (oldest pigs were 10 days old), vaccination (for Mycoplasma, APP, toxigenic Pasteurella and PRRS) and medication (doramectin, ceftiofur, lincomycin and tiamulin). Since the buildings were old and would have needed major repairs anyway, it was decided to build a new farrow-to-finish barn on the same site, about 75 metres from the existing facilities, that would receive the ‘clean’ piglets weaned within the programme.
Following a strict biosecurity protocol, these piglets would then be raised without any direct or indirect contact with the infected population, and become the new sows and boars of that herd. The reason why the owner opted to try such a complicated and risky programme is because genetics in his herd were of great value, and he wanted to preserve that.
The programme was a success, and all five organisms were eliminated from the herd. Table 1 shows the lesions in pigs from that herd before and after the programme, for periods of six months each. As can be seen, the picture was dramatically changed. The first clean piglets were produced in January 1999. Ten years later, the lesions were still at very low levels.
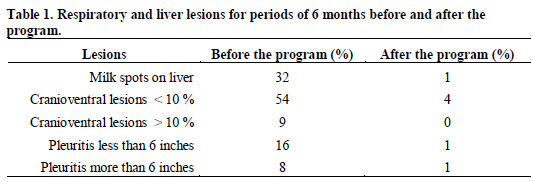
The same spectacular results were obtained as far as antibiotics usage is concerned. Before the programme, the approximate quantity of antibiotics used in the feed was about 80g per pig, while no antibiotics at all were used in the feed, or in the water, after completion of the programme. After the programme, the mortality in that herd was about 2.5 per cent from weaning to slaughter. It should be mentioned that the herd is not located in a hog-dense area, and is 4km away from the next pigs.
One might say that such health improvement programmes are limited to farms located in areas with few pigs but there are herds in France located in very hog-dense areas that have maintained a high health status over many years. These are farms using HEPA air filtration with positive pressure. While this particular air filtration system is very expensive, cheaper alternatives have been developed, and hopefully the search for effective and even cheaper systems will continue.
Management
A 250-sow herd produces 25-kg pigs that are raised in two different finishing sites. On the first site, the small 500-place capacity building (Barn A) is filled in one month but usually emptied before the next batch of pigs is introduced, so it is an all-out system by building. On the second site, the barn is too big (2000 places; Barn B) to be run all in-all out by building with such a small source of piglets, so pigs are introduced every week in an all in-all out system by room.
Table 2 shows the results in these two finishing units in terms of lung lesions at slaughter, mortality and need for added medication (Miclette J, personal communication, 2011).
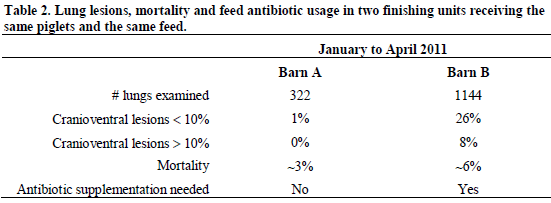
The results obtained are all in favour of Barn A, for which no antibiotic supplementation is necessary. A diagnostic investigation revealed that PRRS virus was circulating in Barn B but not in Barn A. Since Barn B is never emptied, viral circulation is maintained even though the sow herd supplying the piglets produces PRRS-negative pigs.
Similarly, the levels of cranioventral lung lesions in Barn A and in Barn B strongly suggest that Mycoplasma hyopneumoniae is circulating in Barn B but not in Barn A.
In essence, different ways of managing the same pigs produce different results in terms of health status and in terms of need for antibiotic supplementation.
Environment
A farm in England had a problem with Streptococcus suis meningitis (Smith, 2012). Treatment with in-water and injectable potentiated amoxycillins along with the reduced performance and increased mortality of weaners was proving costly.
It was decided to modify the weaners’ environment to reduce the dependence on medication. The main stressor suspected of triggering an outbreak was chilling and temperature fluctuations. An artificial ceiling was constructed from tarpaulin on a roller with an incline of 15 degrees in the autumn. The temperatures in the weaners’ sleeping area became warmer and less daily fluctuations were observed.
The number of treatments, which was 2,309 on average in 2009 and 2010, dropped to three in 2011.
Similarly Holtcamp (2002) reported that in a US farm nursery mortality, which was mainly associated with S. suis, went from almost nine per cent to 2.5 per cent following improvements made in the nursery environment. A vigorous medication programme had shown limited success but the change following environment modifications was dramatic. The changes allowed for continuous air exchange and the elimination of some potential drafts.
Weaning age
This one is quite straightforward. Alban et al. (2010) reported that in Denmark, antibiotic consumption in weaner pigs was 6,692kg for respiratory conditions, and 23,840kg for enteric conditions. So more than three times the quantity of antibiotics is used to control diarrhoea compared to that used to control respiratory diseases.
It is well known that the younger weaning age is, the more likely pigs are to develop diarrhoea post-weaning. In that respect, weaning pigs at an older age is clearly a way to reduce the need for antibiotics.
In a paper presented at the 2012 AASV meeting and involving a challenge with an F18 strain of Escherichia coli at 26 days of age, piglets weaned at 16 days developed diarrhoea earlier and more severely than pigs weaned at 20 days of age (McLamb, 2012). Furthermore, gain reduction was much more pronounced in pigs weaned at 16 days of age (89 per cent) compared to those weaned at 20 days (18 per cent).
Finally, weaning older and heavier pigs can not only have a benefit in terms of antibiotic usage but also in terms of performance. Kim et al. (2012) recently reported that within the same herd, the 70-day weights of pigs weaned at 14, 21 or 28 days of age were respectively 17.48, 26.13 and 28.17 kg, and their feed efficiencies 2.21, 1.41 and 1.24.
Feed
There is a multitude of papers reporting the positive impact of various feed ingredients as alternatives to antibiotics.
In one of them, Evelsizer et al. (2010) used essential oregano oil to prevent problems associated with what we were calling hemorrhagic bowel syndrome, and/or intestinal torsions. For 14,090 control pigs the mortality associated with these problems, which are often prevented using low to moderate antibiotic levels, was 1.29 per cent, while it was only 0.16 per cent for 17,923 pigs receiving the oregano based product. Buddle (2002) has suggested that haemorrhagic bowel syndrome and intestinal torsions were different manifestations of the same condition, and proposed a different name: porcine intestinal distension syndrome.
Season
The Iowa State University Veterinary Diagnostic Laboratory maintains data of the various diagnostics made each year. In an eight-year compilation (2003-2010) of enzootic pneumonia diagnostics, it was found that the number in September-October was close to three times what it is between February and June (Schwartz K, personal communication, 2011).
This suggests that there are situations where instead of using antibiotics year-round to control that condition, there may be opportunities to design antibiotic programmes according to the relative seasonal risk - in other words, a reduced antibiotic usage in periods of reduced risks.
Genetics
In the author's opinion, this is an area where we are likely to make significant progress in the coming years.
In an evaluation conducted in Denmark, 12 Duroc boars, 700 sow and 12,268 pigs were used. The pigs from the different boars were born and raised in the same three farrow-to-finish farms that had been selected because they had respiratory disease problems (Nielsen et al., 2006).
It was found that in the nursery phase, one of the boars produced piglets that had a mortality rate of three per cent while it was 10 per cent for another one. Similarly, in the finishing phase, one boar produced pigs with a mortality of two per cent while another one had a 10 per cent mortality in its progeny. The mortality differences between boars were highly significant statistically (p<0.002 in the nursery and p<0.0007 in finishing).
Differences between the progeny of boars were also found in pleuritis (44 per cent for the best and 68 per cent for the worst; p<0.0001) and pneumonia (18 per cent for the best and 57 per cent for the worst; p<0.0001).
These results, where pigs from different boars were raised side-by-side in the same environment clearly indicate that some boars produce pigs that have a better survivability and resistance to disease than others. Using such genitors would evidently increase the possibilities of raising pigs with reduced dependence on antibiotics.
Gender
Pommier et al. (2008) found differences between genders in the percentage of lungs with pleuritis (9.1 per cent for females, 11.0 per cent for males; p=0.02) and pneumonia (54.4 per cent for females and 61.9 per cent for males; p<0.001) at slaughter.
In a study involving six finishing units and 7,982 pigs, 68 per cent of the pigs that died were males and 32 per cent were females (Surprenant C, personal communication, 2011).
In another study involving close to half a million pigs, mortality in gilts receiving no antibiotics was at 4.26 per cent, while it was 6.41 per cent for barrows receiving antibiotics (Moreau, 2001).
Since females are less sick and survive better than males, raising them separately increases the possibilities of reduced antibiotic usage for at least half the production.
Furthermore, a recent study by Jungst et al. (2012) showed that due to the difference in top quality pigs and feed cost savings, there was an advantage of $4.22 per pig when barrows and gilts were raised separately, compared to raised together.
Parity segregation
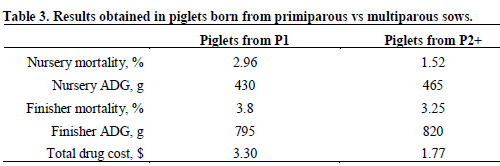
Moore (2003) reported on a strategy called parity segregation where piglets born from gilts are raised separately from piglets born from sows of other parities. Piglets born from gilts are more susceptible to different conditions and may thus require more aggressive preventive control programs.
Table 3 shows the results that were obtained. All parameters evaluated, including drug cost, were improved in piglets born from multiparous sows. Parity segregation thus offers the possibility to reduce antibiotic usage for about 75 per cent of the pigs produced.
Individual treatments
One of the reasons why Denmark is able to produce pigs with less antibiotics than many other countries is that they rely much less on treatment of the whole population than, for example, in North America. They will treat individual pigs, or pens, but generally not the whole group of pigs.
In some situations, treating individual pigs has been shown to produce better results, at lower costs than treating all the animals with antibiotics in the feed or water.
Table 4 shows the results that were obtained in a Quebec company where pigs affected with porcine pleuropneumonia were either treated individually by injection, or the total group was treated in the feed and/or water (Desrosiers, 1986).

Not only were the results obtained clearly better with individual treatments but the antibiotic cost associated with it was also much lower. It should be mentioned that when this study was conducted, the choice of antibiotics that could be used in the feed or water was more limited than it is today. Nevertheless, particularly for acute conditions like porcine pleuropneumonia that reduce feed and water consumption of affected animals, injection of sick pigs is often more cost-effective than oral treatment of the whole group.
Vaccines
Obviously, using vaccines to prevent disease rather than antibiotics is an easy way to reduce antibiotic usage.
In a study conducted in Denmark on 20 farms, those using a live Lawsonia intracellularis vaccine used less antibiotics than those that did not (11.4 ADD per 100 weaned pigs versus 14.8) (Bundgaard, 2012).
Single source vs multi-source
Martano et al. (2012) have reported the mortality rates obtained in finishing units in Italy depending on whether the piglets came from one source or more. The data were compiled over the period of January 2006 to December 2010, and involved 454,620 pigs.
The average mortality rate of single-source pigs was 3.43 per cent against 5.60 per cent for those coming from more than one source, and the difference was statistically significant (p<0.05). The reduced mortality rate in single-source pigs shows that this way of raising pigs is less likely to be a problem on a health basis, and thus has the potential to reduce the need for medication.
It should be kept in mind, however, that there are situations where it may be preferable to introduce more than one source of pigs on a site, if it allows this site to work on an all in-all out basis by site, rather than by room or building.
Try and see
Brumm et al. (2012) compared groups of pigs that received five different medication programmes in the feed to pigs that had no medication. The study involved 1,800 pigs that were evaluated from day 45 to 159 post-weaning. One of the medication programs included the following antibiotics and schedule: chlortetracycline and tiamulin for two weeks, then chlortetracycline for two weeks, then tylosin for two weeks, then chlortetracycline and tiamulin for two weeks, then tylosin up to slaughter. Another one included tylosin from the beginning to the end at decreasing dosages.
There was no effect of any of experimental treatments on the number of pigs dead, pigs removed or morbidity as measured by the number of pigs given injectable treatments.
Table 5 shows the results that were obtained in terms of average daily gain (ADG) and feed per gain (F/G) for the unmedicated control group and the five medicated groups.

There was no significant difference in either average daily gain (ADG) or feed:gain ratio (F/G) results. The group receiving non medicated feeds did as well as the other ones. This shows that including antibiotics to feeds, particularly in the finishing units, does not automatically result in an improved performance.
Producers who are not convinced that a medication program installed a while ago is still cost effective could try to remove or reduce that medication programme and see if it does or does not produce the expected benefits.
Other options
There are other options to reduce antibiotic usage in swine production.
Improving biosecurity in a way that allows reducing the introduction of new pathogens or strains in a given herd is an obvious one, the PRRS virus being an easy example of that.
Producers have a tendency to think that if a little is good, more is better, and there are cases where the dosage used to treat a condition is much higher than necessary. Some antibiotics can be effective at dosages (mg of antibiotic per kg of pig) that are a lot lower than others, so switching to these products automatically reduces the grams of antibiotics per pig, but in this case other issues obviously need to be considered.
Using pulse treatments rather than continuous medication can not only reduce the total dosage of antibiotics used but may also allow the pig to mount an immune response to the organism targeted.
In a recent study, electrostatic particle ionisation was shown to significantly improve air quality, gain and mortality of nursery pigs and may thus reduce antibiotic usage by allowing raising healthier pigs (Rademacher, 2012).
The options described in this paper are not inclusive, and we should keep an open mind to any product, strategy or technique that has the potential to reduce antibiotic usage in our production.
Choice of Antibiotics Used
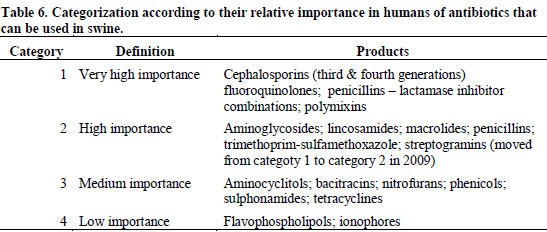
Antibiotic usage per se is only one aspect of the problem. The choice of antibiotic used is another important one. Some antibiotics are considered as the last resort therapeutic compounds against certain human and swine diseases, and it is of paramount importance to make sure that these antibiotics remain effective on a long-term basis. This is particularly true when considering that access to new antibiotics is, as mentioned before, likely to be rather limited in the future. For that reason, their usage should be limited to situations where they are really necessary.
The Canadian Veterinary Medical Association produced a document in which it categorized the various antibiotics available according to their strategic importance in human medicine (Agriculture and Agri-Food Canada, 2008). This categorisation is shown in Table 6.
Conclusions
To what extent antibiotic usage in animals is responsible for the developing resistance of human pathogens to some key antimicrobial molecules remains a topic of vivid discussions.
Some say it is of marginal importance, others state that it is significant and that it would be irresponsible not to address it. But which side serves us better?
Putting energy, time and money trying to prove that the use we make of antibiotics in animals is ‘not that bad’, or changing our ways so that the human medical authorities appreciate the effort we make to address the issue and eventually become more collaborative and conciliatory with livestock producers and animal health professionals?
Independently of pressures from the human side, we need to keep in mind that increasing antibiotic resistance to swine pathogens is also a concern.
Pigs can get sick, and when they do, they need to be treated effectively and this will be compromised if antibiotic resistance to some important pathogens continues to increase.
Finally, producing pigs that are raised with less antibiotic usage is something that can be used as a comparative advantage, when it comes time to offer Canadian pork to local and export markets.
A limited and judicious use of antibiotics in swine production would thus seem to be in the best interest of all those involved in our industry.
References
Aarestrup F, et al. 2010. Changes in the use of antimicrobials and the effects on productivity of swine farms in Denmark. Am J Vet Res. 71:726-733.
Agriculture and Agri-Food Canada. 2008. Canadian veterinary medical association antimicrobial prudent use guidelines 2008.
Alban L, et al. 2010. Forbrug af antibiotika til svin. Dansk Veteri. 15:10-15.
Alban L, et al. 2012. Possible impact of the “yellow card” antimicrobial scheme on meat inspection lesions in Danish finisher pigs. Prev Vet Med. Epub ahead of publication.
Bondt N, et al. 2012. Trends in veterinary antibiotic use in the Netherlands 2005-2011. www.maran.wur.nl June 15.
Brumm M, et al. 2012. Impact of in-feed antibiotic regimens on pig performance and expression of clinical and subclinical diseases. Proc AASV. 277-281.
Buddle JR, et al. 2002. The porcine intestinal distension syndrome. Pig J. 50:68-82.
Bundgaard H, et al. 2012. How to reduce antimicrobial use in pig production. Proc IPVS. Vol 1, 200.
Chan M. 2012. The EU’s contributions to the solutions of the global antimicrobial resistance problem. Conference on antimicrobial resistance, Copenhagen, March 14-15.
Desrosiers R. 1986. Therapeutic control and economic aspect of porcine pleuropneumonia in finishing units. Vet Rec. 119:89-90.
Desrosiers R. 2001. How to get rid of PRRS, pleuropneumonia, enzootic pneumonia, atrophic rhinitis and mange… at the same time! Int Pig Letter 21:22-23.
Evelsizer RW, et al. 2010. A field experience using oregano essential oil in herds with hemorrhagic bowel syndrome. Proc AASV. 419.
Holtcamp A. 2002. Environmental controls of Streptococcus suis meningitis cases. Proc ISU Swine Dis Conf Swine Vet. 23-24.
Jungst S, et al. 2012. Mixed-sex vs split-sexed penning. Does it matter at the commercial level? Proc IPVS. Vol 1, 149.
Kim KH, et al. 2012. Effect of weaning age on growth performance and nutrient digestibility in piglets. Proc IPVS. Vol 2, 572.
Luppi A, et al. 2012. Antimicrobial resistance of swine Escherichia coli F4+ strains isolated in Italy from 2002 to 2011. Proc IPVS. 79.
MAPAQ. 2012. Rapport annuel 2011. Surveillance de l’antimicrobiorésistance. www.mapaq.gouv.qc.ca/antibioresistance
Martano G, et al. 2012. Research on mortality rate in fattening pig herds. Proc IPVS. Vol 2, 815.
McLamb et al. 2012. Early weaning impairs gut mucosal defenses and exacerbates clinical disease in F18 E coli infection. Proc AASV. 91-95.
Moore C. 2003. Parity segregation, successes and pitfalls. ISU Swine Dis Conf Swine Prac. 47- 52.
Moreau I, et al. 2001 Observations and description of an intervention method affecting death loss rate in all in/all out finishers. Proc AD Leman Swine Conf. 197-200.
Moreno A. 2012. Antibiotics in animal feed. Consumer ReportsÒ National Research Center, April.
News. 2012. Danish pig industry reduced antibiotic usage by 19 per cent in 2011. Pig Progress.net, Feb 29.
Nielsen B, et al. 2006. Genetic effects of survival and lung diseases in growing pigs. Genetics applied to livestock production, 8th World Congress.
Park J, et al. 2013. An investigation of exudative epidermitis (greasy pig disease) and antimicrobial resistance patterns of Staphylococcus hyicus and Staphylococcus aureus isolated from clinical cases. Can Vet J. 54:139-144.
Pommier P, et al. 2008. Sex is a risk factor for lung lesions of pigs at slaughter. Proc IPVS. Vol 2, 389.
Rademacher C. et al. 2012. Electrostatic particle ionization (EPI) improves nursery pig performance and air quality. Proc AASV. 257-258.
Sibbald B. 2012. Farm-grown superbugs: While the world acts, Canada dawdles. Can Med Ass J. 184:1553.
Smith JL. 2012. Reducing the incidence and treatment of meningitis caused by Streptococcus suis in weaner pigs by adapting the environment. Proc IPVS. Vol 1, 250.
Stege H, et al. 2010. Monitoring of antibiotic usage for pigs in Denmark. Proc IPVS. Vol 2, 1005.
US Food and Drug Administration. 2012. Cephalosporin order of prohibition goes into effect. April 6.
Reference
Derosiers R. 2013. Why we should reduce antibiotic usage and ways to do it. Proceedings of the London Swine Conference, London, Ontario, Canada. March 2013. p105-116.
September 2013







