



Addressing Antibiotic Resistance in Swine
Reducing antibiotic resistant bacteria in swine farms positively influences swine production and helps address problems of antibiotic resistance and residues in meat. Reduced resistance to pathogenic E.coli contributes to successful antibiotic treatment during disease outbreaks, writes Nataliya Roth, Product manager, Acidifiers with Biomin.Worldwide, antibiotics are used in animal production at therapeutic levels for the treatment of infections and for growth promotion or prophylaxis. The disadvantage of antibiotics is the emergence and spread of resistant bacteria. Resistant bacteria have become a major concern for both animal health and the public as human medicine is running out of antibiotics that are still effective in treating certain infections.
Antibiotic use in animal production has been identified as a risk factor in the development of antibiotic resistant bacteria that can be transferred to humans via several routes. These include the consumption of animal products, exposure to resistant microorganisms from contact with animals, and the contamination of ground and surface waters by wastes containing antimicrobials and resistant microorganisms.
Exposure to antibiotics not only increases the level of antibiotic resistance among bacteria belonging to the normal intestinal flora of animals but also among pathogenic bacteria. Where high levels of resistant pathogenic bacteria are present, antibiotic treatments may no longer be effective against pathogens.
| It is necessary to reduce the use of antimicrobial drugs to control antibiotic resistance. Another way is to reduce the levels of resistant bacteria in the gastrointestinal tract of animal. This lowers the load of resistant bacterial in the environment, which consequently reduces the transmission of resistance genes. Lowering antibiotic resistance is especially important for those bacterial species common in humans and animals. For example, the bacteria E. coli found in food is ingested by humans every day. As antibiotic-resistant strains of E. coli are ubiquitous in both human and animal isolates, E. coli is used as an indicator for resistance problems in both animals and humans. |
E. coli Resistance in Swine
Surveillance and monitoring studies on antimicrobial resistance provide information about the occurrence of resistances in pigs in different parts of the world. E. coli resistance in swine was described in the Austrian Resistance Report AURES, a yearly report published since 2004 on the levels of resistance in humans and the veterinary sector.
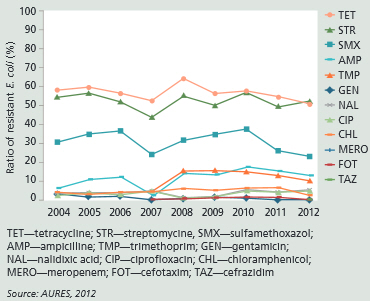
To date, a total of about 160 digesta samples from the large intestine of swine from 30 farms in Austria have been analysed for E. coli. Tests for antimicrobial susceptibility to different antibiotics were conducted. The microbiological resistance of E. coli using epidemiological cut-off values is shown in Figure 1.
Epidemiological cut-off values are determined on the basis of the distribution of minimal inhibitory concentration for an antibiotic and a bacterial species. The cut-off values for different antibiotics are presented by the European Committee on Antimicrobial Susceptibility Testing (EUCAST).
Determining Resistance
The ratio of resistant E. coli was determined as follows:
[counts of resistant E. coli per year/ counts of tested E. coli per year] × 100.
Ratios of E. coli resistant to tetracycline, streptomycine, sulfamethoxazol and ampicillin were between 15 and 50 per cent in 2012. These percentages were higher than the ratio of E. coli resistant to other tested antibiotics. For this reason, E. coli resistance to tetracycline, streptomycine, sulfamethoxazol and ampicillin were determined in the following swine trial. Multi-resistance includes resistances to tetracycline, streptomycine and sulfamethoxazol (Tet+Str+Sul).
Swine Trial
A trial with weaned pigs showed that it was possible to minimise the incidence of resistant bacteria and reduce the number of multi-resistant bacteria in the gastrointestinal tract of swine with the help of a combined feed additive. This feed additive consisted of organic acids, cinnamaldehyde and a permeabilizer (OCP) in the form of a commercial product, Biotronic® Top3 (Biomin).
The trial was carried out at the Biomin Center of Applied Animal Nutrition in Mank, Austria, using 60 pigs [(Landrace × Large White) × Pietrain]. Pigs, two weeks after weaning (bodyweight 12.27kg; 40 days) were assigned to two treatments. The negative control group diet contained no growth-promoting feed additives, whereas the diet of the trial group was supplemented with Biotronic Top3 at the inclusion rate of 1.0kg per tonne of feed. No antibiotics were added to the feed.
The duration of the trial was 42 days. Body weight and feed intake were recorded, and feed conversion ratio was calculated. Faecal samples of 16 pigs per pen were collected and immediately frozen on day 14 and 42. The counts of E. coli as well as E. coli resistant to ampicillin and multi-resistant to Tetr+Str+Sul were determined in all faecal samples. The results of the trial are shown below.
Fighting Bacterial Resistance
By reducing antibiotic resistant bacteria, natural feed additives provide a possible solution to the global problem of antibiotic resistance, as the swine trial shows. Moreover, the reduction in opportunistic pathogens and antibiotic resistant bacteria minimises the risk of infections among animals and positively influences swine production.
The lower the counts of resistant bacteria in the intestinal flora, the lower the possibility that genes encoding resistance will be transferred to other bacteria including pathogenic bacteria. This will also reduce the dissemination of resistant bacteria in the farm environment. Reducing the resistance of pathogenic E. coli to antibiotics contributes to the successful treatment of animals during a disease outbreak.
Results
Improvements in body weight and weight gain were seen in the group that received diets supplemented with Biotronic® Top3 (Table 1). Bodyweight at day 42 was three per cent higher in the trial group compared to the control group. Average weight gain in the Biotronic Top3 group was four per cent higher than the control group.
| Table 1. Performance characteristics of piglet | ||||||||
| Period | Bodyweight (kg) | Weight gain (kg) | Feed intake (g) | FCR | ||||
|---|---|---|---|---|---|---|---|---|
| Control | Biotronic | Control | Biotronic | Control | Biotronic | Control | Biotronic | |
| Day 14 / Period 1-14 | 19.25 | 19.46 | 6.97 | 7.20 | 725 | 761 | 1.58 | 1.58 |
| Day 28 / Period 1-28 | 29.10 | 29.27 | 16.83 | 17.00 | 974 | 1,014 | 1.62 | 1.67 |
| Day 42 / Period 1-42 | 37.71 | 38.75 | 25.45 | 26.48 | 1,110 | 1,161 | 1.83 | 1.84 |
Analysis of samples on day 14 showed no difference in the total E. coli count between groups but lower counts of resistant E. coli in the group fed Biotronic Top3 (Table 2).
| Table 2. E. coli in faecal samples on day 14, cfu/ml | ||||||||
| Control | Biotronic | Average | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 | Control | Biotronic | |
| E. coli | 1.14E+07 | 5.50E+04 | 2.08E+07 | 5.91E+05 | 3.62E+06 | 2.90E+07 | 1.08E+07 | 1.11E+07 |
| E. coli resistant to Tetr+Str+Sul |
7.64E+06 | 4.00E+03 | 4.06E+06 | 6.55E+05 | 7.66E+04 | 3.55E+05 | 3.90E+06 | 3.62E+05 |
| E. coli resistant to Ampicillin |
7.20E+05 | 9.00E+03 | 8.56E+05 | 0.00E+00 | 1.98E+05 | 4.42E+05 | 5.28E+05 | 2.13E+05 |
Microbiological analysis at the end of the trial (day 42) showed that total E. coli counts in the faecal samples of the group fed Biotronic Top3 was about 90 per cent lower than the control group (Table 3).
| Table 3. E. coli in faecal samples on day 42, cfu/ml | ||||||||
| Control | Biotronic | Average | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 | Control | Biotronic | |
| E. coli | 2.20E+05 | 1.73E+06 | 1.59E+06 | 1.33E+05 | 1.13E+05 | 1.50E+05 | 1.18E+06 | 1.32E+05 |
| E. coli resistant to Tetr+Str+Sul |
9.41E+03 | 6.62E+03 | 2.01E+05 | 4.29E+02 | 1.82E+03 | 4.39E+03 | 7.23E+04 | 2.21E+03 |
| E. coli resistant to Ampicillin |
5.17E+04 | 6.62E+03 | 3.71E+05 | 4.18E+04 | 4.68E+03 | 1.15E+04 | 1.43E+05 | 1.93E+04 |
The count of E. coli resistant to ampicillin in the trial group was 60 per cent below the control group.
The count of E. coli with multi-resistance to Tre+Str+Sul in the trial group was nearly 90 per cent below the control group.
Figure 2 shows the average E. coli and resistant E. coli counts in the faecal samples of pigs at the end of the trial
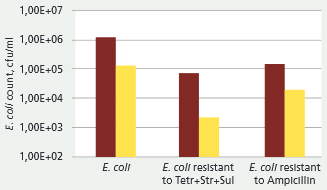
[Brown bars: average control; yellow bars: average OCP]
June 2014






