



Current Antimicrobial Use and Resistance Issues in the European Union - Reality and Resolution
The European Commission has put forward a 12-point programme aimed to reduce the use of antibiotics in veterinary medicine. Dr David Burch of Octagon Services in the UK explained the background and likely developments in a paper he presented as The 'Dr Roy Schultz Lecture' at Iowa State University in November 2012].Introduction
In the European Union (EU) over the last 40 years, there have been a number of scares regarding antimicrobial
resistance and the way antimicrobials have been used. Most of these scares have originated in human medicine.
But as a result, the use of antimicrobials in animal health also has come under scrutiny, either as a possible
source of the problem or an exacerbation of an existing one.
This has resulted in the introduction of a variety
of restrictions over the use of antibiotics in the EU, especially in comparison with the United States. With
the recent rise in methicillin-resistant Staphylococcus aureus (MRSA) in pigs and the emergence of extended-spectrum
beta-lactamase (ESBL) producing Escherichia coli, especially in chickens but also in pigs and cattle,
these have caused the European Commission (EC) to review the legislation regarding the use of veterinary
medicines and in particular the use of antibiotics.
At the time of the writing of this paper, there is ongoing
consultation regarding the EC's 12-point plan1 regarding antimicrobial resistance issues and the actual
problems and their possible resolutions.
The European Union: Decision-Making
The decision-making process in the EU is complex, and although it is meant to be by consensus, after full
consultation, there are potentially a number of pitfalls along the way. Outcomes can be difficult to predict.
The EU is currently a complex, loose federation of countries, unlike the United States. Initially, it was set up to
harmonise trade but also as a defense against a further world war and against the communist threat in the East,
at that time. Now it is a group of 27 countries, including many of the former Eastern European countries, and
with 17 countries linked together economically by a common currency forming the 'Eurozone'. Each country
currently is independent with its own government and laws, often speaking a different language but agreeing to
comply with the EU rules and umbrella set out by a number of EU treaties.
Regulations and directives are established and implemented by the EC, with which the member states have to
comply, hence its significance. It is a non-elected administrative body with a president and with heads of the
various directorates general appointed from a variety of countries. DG Sanco (Health and Consumer Affairs)
is responsible for medicine issues. The European Parliament (EP) is an elected body and makes recommendations
to the commission, and the European Council is made up of the heads of the member states under its
president and also plays an important role as the 'political strategist'.
To help with the decision-making regarding implementation, the Commission has its own EU groups and
organisations that they consult, then drawing information from their 'experts'. Of importance regarding
medicines are the European Medicines Agency (EMA), responsible for human and veterinary medicines
(CVM equivalent), and the European Food Safety Authority (EFSA), responsible for coccidiostats and
histomonostats, formerly growth promoters and other non-prescription feed additives such as probiotics, etc.
The European Centre for Disease Control (ECDC) monitors disease situations on the human and animal side.
Other animal-associated bodies of importance are the International Federation for Animal Health (IFAH)
Europe (pharmaceutical industry) (AHI equivalent), the Federation of Vets in Europe (FVE) (AVMA equivalent), the European Platform for Responsible Use of Medicines in Animals (EPRUMA), and farmer and cooperative
associations (COPA-COGECA).
Member states take turns setting the agenda for the council and rotate every six months, with Denmark being
in charge for the first half of 2012. They have played a very strong role in the anti-antibiotic debate and held
a conference in Copenhagen in March 2012, highlighting many of the problems of antimicrobial resistance in
man and animal.
Historical EU Resistance Issues
In 1969 in the UK, the Swann Report2 recommended that antibiotics that 'have little or no application as
therapeutic agents in Man or animals and will not impair the efficacy of a prescribed therapeutic drug or drugs
through the development of resistant strains of organisms' should be usable for growth promotion. The list of
unsuitable antibiotics at that time was chlortetracycline, oxytetracycline, penicillin, tylosin (a macrolide related
to erythromycin), and the sulphonamides.
In the UK and most of Europe, these antimicrobials were put under veterinary control on prescription. Only tylosin had a dual role as growth promoter and prescription product. In the United States, they all remained mainly prescription-free.
Over time, the list of growth promoters grew to include carbadox, olaquindox, avilamycin, avoparcin,
flavophospholipol, oleandomycin, salinomycin, monensin, tylosin, virginiamycin and bacitracin - mostly
drugs not considered of importance or in use for human medicine.
Sweden banned growth promoters in 1986. In the mid-1990s, concern arose in human medicine about the
growth promoter, avoparcin, as it was related to the glycopeptide vancomycin, which was increasingly being
used for the treatment of MRSA. The drive to ban growth promoters in the EU came from the concern
regarding the development of vancomycin resistance in man, particularly in enterococci (VRE). Avoparcin
had been used in pigs and poultry since its approval in the 1980s3. Denmark banned it in 1995, and under
the 'precautionary principle', it was decided to ban its use in the rest of the EU in 1997, as avoparcin-resistant
enterococci had been determined in pigs and poultry.
It was thought possible that this resistance could be transferred to Man via contaminated food/meat products.
This sounded reasonable and logical at the time, but the link or the extent of the risk was never quantified. In
the United States, avoparcin was not approved for use in animals, yet they had the most severe VRE problems
in Man4, mainly in hospitals at the same time, and even had vancomycin-resistant S. aureus (VRSA)5. It is
postulated that the resistance epidemic arose from the increased use of vancomycin in human patients to
combat MRSA and enterococcal infections, following the massive increase in immunocompromised patients
with human immunodeficiency virus (HIV) or who were being treated with chemotherapy for cancer or to
stop organ transplant rejection.6
Virginiamycin, a streptogramin, is related to quinupristin/dalfopristin, another product used in Man for
MRSA, and was withdrawn on the same 'precautionary' basis that resistance might be transferred via faecal
enterococci. Denmark banned virginiamycin in 1998 and the EU in 1999, along with bacitracin and the
macrolides, tylosin and spiramycin. Tylosin reverted back to being a prescription-only medicine.
In 2006, all
growth promoters were banned, yet monensin and salinomycin, both ionophore antibiotics, are still widely
used in poultry production as coccidiostats. Carbadox, virginiamycin, flavophospholipol, avilamycin, and
bacitracin are still used in pigs in the United States. The early ban on growth promoters in Denmark caused a
dramatic increase in the use of therapeutic antibiotics in response to increased enteric disease such as
colibacillosis and ileitis.
Only tylosin was evaluated for the risk of treatment failure in Man, associated with macrolide-resistant
E. faecium from pigs, and the risk was considered very small with a probability of 1 in 21 billion7. Using
macrolide resistance as a marker, the risk of Campylobacter coli infection in pigs and any resistance being
spread to Man also were considered low to zero.8
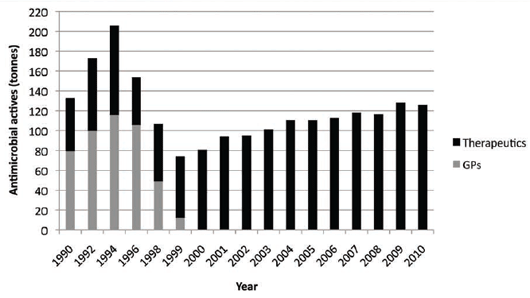
The use of the 'precautionary principle' has really caused great suspicion over decisions made by the European
Commission at the time, as in hindsight, it was primarily a theoretical and political decision9, possibly driven
by the Scandinavians rather than being science-based. Regarding resistance issues, there has been minimal
impact on improving human health following the ban and initially, there were some serious health and animal
welfare issues, which resulted in an increase in the use of prescription-only antimicrobial medicines10 (see
Figure 1).
Current EU Resistance Issues
Methicillin-Resistant Staphylococcus aureus (MRSA)
In 2005, the occurrence was reported of a new clonal MRSA (CC398) in pigs in The Netherlands11 and
subsequently its widespread prevalence12. A detailed survey was carried out across the EU13,14 by EFSA in
most member states, and it was shown to be widespread in pigs. The spread of clones across Europe also was
linked to pig movement, whether for breeding or for finishing. Some countries, such as the UK, remained free
of the infection, possibly due to them being primarily exporters of breeding pigs and importers of meat rather
than live animals. In addition, the routine prophylactic use of third-generation cephalosporins, such as
ceftiofur, at processing was not common, as 40 per cent of the UK herd is reared outdoors and male pigs are
not castrated.
In spite of this, the UK was going through a major MRSA epidemic but it was linked primarily to the medical
arena and poor hospital hygiene practices15, 16 (see Figure 2).
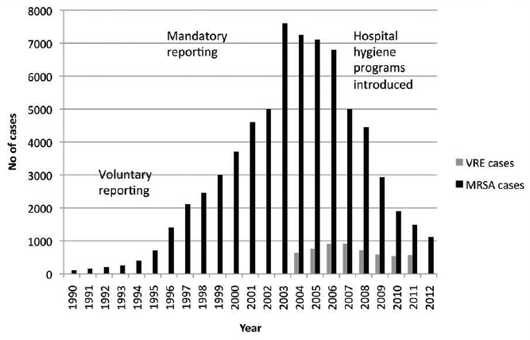
The introduction of hospital hygiene initiatives has resulted in a dramatic fall in the incidence of MRSA cases
in England and possibly a halving of VRE cases.
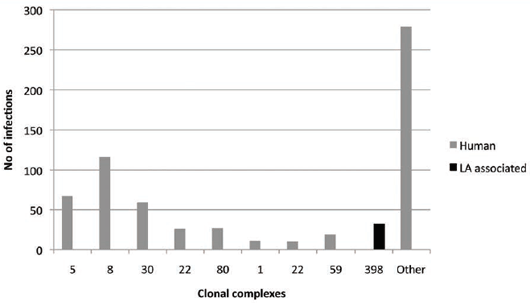
In other countries, MRSA CC398 has been found in Man primarily in people associated with agriculture (e.g. farmers, their families, slaughterhouse workers and veterinarians). Spread beyond to the wider general public
has appeared minimal17. In Denmark, 4.95 per cent of 646 human MRSA infections were associated
with CC398, the livestock-associated (LA) clone in 201010 (see Figure 3). Asymptomatic carriers were
higher at 13.53 per cent of 451 isolates.
Overall, MRSA CC398 played a 9.57 per cent role in a rapidly growing problem in Denmark, where new MRSA
cases (both infected and just colonised) increased from approximately 100 in 2001 to 1,097 in 2010 (an 11-fold increase). This has led to a voluntary suspension of use of third- and fourth-generation cephalosporins in
veterinary medicine in 2010 and the implementation of a number of other controls to reduce antimicrobial use.
Although the majority of the MRSA issues are related to human medicine, a proportion of infections can be
attributed to the LA form of MRSA, mainly spa type t011 t034 found in pigs.
Extended-Spectrum Beta Lactamases (ESBLs)
In Europe, there are calls to ban the use of third- and fourth-generation cephalosporins in veterinary medicine.
The United States has already put restrictions on the use of all cephalosporins to 'on-label' use only and in
the EU, the cessation of use is being implemented for third-generation cephalosporins, such as ceftiofur, in
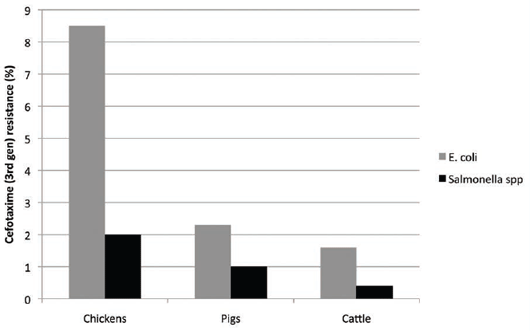
the poultry industry, where they were not licensed for use. There was a high level of ESBLs found in E.coli, on
average 8.5 per cent (range 0–26.4 per cent in chickens in an EFSA survey)18. It was thought to be associated
with the extensive use of injections in-ovo and in day-old chicks and possibly spraying, even though it was not
approved for use. By comparison, ESBL production in porcine E.coli in the EU was low at about 2.3 per cent
(range 0–3.8 per cent)18 (see Figure 4).
Contamination of both broiler and pig meat with ESBL-producing
E.coli also was reported, hence the public health concern. A tightening up of their use has been implemented
by the EMA, but some countries, such as Denmark and France, have voluntarily stopped using them.
Fluoroquinolone resistance
The fluoroquinolone-resistance debate has been ongoing since their introduction into veterinary medicine in
the 1990s. Their use in poultry was banned in the United States in 2005, and Denmark also stopped their use
altogether in the same year. They are exceptional broad-spectrum antimicrobials and have proven very popular
in veterinary and human medicine. Recent resistance issues have been noted in The Netherlands,19 especially
in poultry (see Figure 5).
A much higher level of fluoroquinolone (ciprofloxacin) resistance at the low and high cutoff break point was
seen in E. coli from broilers rather than in swine. Enrofloxacin, the veterinary fluoroquinolone, was widely
used in the drinking water of broilers but primarily by injection in pigs, although there is also an oral-doser
form in the EU. Cefotaxime (third-generation cephalosporin) also was much higher in broilers than in pigs.
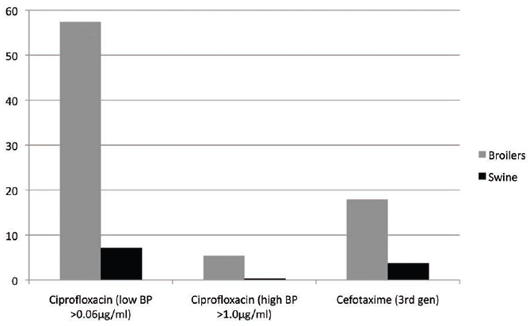
All these issues led to the Dutch authorities introducing stringent controls on the veterinary use of antimicrobials
and a target reduction of antimicrobial use by 50 per cent by 2013 from their peak in 2007. Antimicrobial
feed premixes have been forbidden, and the use of antimicrobials is monitored centrally so that individual
practices are watched for their use. Neither the fluoroquinolones nor the cephalosporins are used in feed in
the EU, and the main impact has been to reduce tetracycline use, which has negligible impact on human
resistance issues.
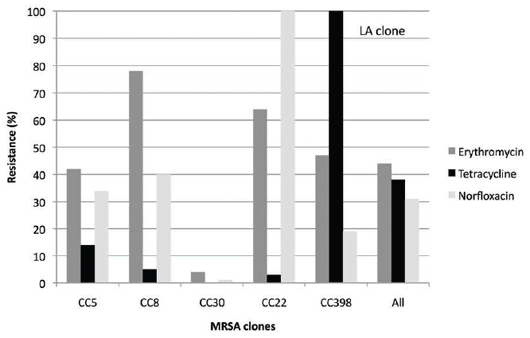
Many of the human Danish MRSA strains also show resistance to fluoroquinolones (norfloxacin) but low
levels of resistance only to tetracyclines, in comparison with LA MRSA CC39810 (see Figure 6).
Antimicrobial Use in Man and animals
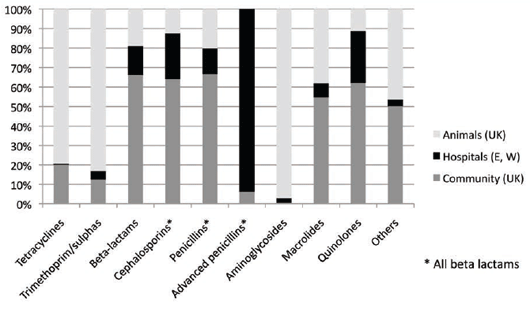
In 2010, the UK's Veterinary Medicines Directorate published figures comparing the use of families of
antimicrobials used in man in England and Wales and also in animals in the UK20 (see Figure 7).
Tetracyclines and trimethoprim/sulphonamide combinations are commonly used in animal medicine,
especially as medicated feed premixes. The beta-lactam antibiotics are widely used in human medicine because
of their relative safety and efficacy, as well as the fluoroquinolones. The use of aminoglycosides in Man also has
fallen away due to nephro- and oto-toxicity problems.
In the EU, they are starting to coordinate antimicrobial use in member states. The European Surveillance of
Antimicrobial Consumption (ESVAC) project has been set up by the EMA, initially involving seven EU countries
as well as Switzerland and Norway21. They looked at antimicrobial consumption and correlated it with
the number of animals treated. This data will form a baseline, and the plan is to expand this to include more
member states in forthcoming years to better correlate antimicrobial use with antimicrobial resistance in
the EU.
The Future: Resolution?
The European Commission put forward a 12-point program1 (see Table 1). This will form the basis for further progress, but the details are under discussion at the moment. Not all member states want the harsh conditions for veterinary medicines that are being proposed by Denmark and The Netherlands, yet most feel that advances must be made.
The European 12-point Program.1
- Strengthen the promotion of the appropriate use of antimicrobials in all member states.
- Strengthen the regulatory framework on veterinary medicines and on medicated feed.
- Introduce recommendations for prudent use in veterinary medicine, including follow-up reports.
- Strengthen infection prevention and control in health care settings.
- Introduce a legal tool to enhance prevention and control of infections in animals in the new Animal Health Law.
- Promote, in a staged approach, unprecedented collaborative research and development efforts to bring new antimicrobials to patients.
- Promote efforts to analyze the need for new antibiotics into veterinary medicine.
- Develop and/or strengthen multilateral and bilateral commitments for the prevention and control of AMR in all sectors.
- Strengthen surveillance systems on AMR and antimicrobial consumption in human medicine.
- Strengthen surveillance systems on AMR and antimicrobial consumption in animal medicine.
- Reinforce and coordinate research efforts.
- Survey and comparative effectiveness research.
The European Council has recently endorsed much of this work22 with a strong belief in 'One Health' between Man and animals. It has called on member states to develop and implement national strategies or action plans for countering antimicrobial resistance. This includes:
- Prudent-use guidelines, clinical sampling and susceptibility testing, and education and training of professionals.
- Illegal sales of antimicrobials will be stopped, especially over the Internet.
- Limit the use of critically important antibiotics to cases in which diagnosis and susceptibility testing have determined their use.
- Limit prophylactic use of antimicrobials to cases of defined clinical needs, and limit herd treatments to cases in which a veterinarian has assessed a clear clinical justification to treat all animals.
- Encourage animal production and marketing systems that serve the continuous improvement of animal health to reduce the need for antimicrobial use.
- Ensure effective surveillance systems in both the human and veterinary sectors on the use of antimicrobials and resistance, and collect sales and use data for both.
- There should be intersectoral coordination.
They also called upon the Commission to work with the member states on the above but also to raise public awareness about the risks of antimicrobial resistance and to work with international agencies such as the WHO, OIE and Codex. They also supported the global ban of antimicrobial growth promoters and wanted the commission to look into the prescription and sales of antimicrobials and whether this led to over-prescription, overuse, or misuse. The Council also wanted a review of medicated feedingstuffs in the EU, taking resistance into account, and that prescriptions for antibiotics only may be carried out by veterinarians.
Conclusions
The debate will surely continue for some time to come. Most of the proposed developments make sense;
however, the devil is in the details, and these are not yet published.
It is hoped to retain access to the fluoroquinolones and third- and fourth-generation cephalosporins by education regarding their prudent use.
Antimicrobial growth promoters have gone but there are attempts to retain antimicrobial feed premixes and also preventive use, which is felt to be critical, especially in pig production.
Veterinarians already have to prescribe antimicrobial products in the EU, unlike the United States but the supply of antimicrobials and the profit from their supply is still a major issue.
There will be more controls, and it is considered just a matter of time before centralised recording of antimicrobial use on a practice and possibly on a farm basis will be introduced, and high-level use may be investigated.
There also needs to be help for farmers to improve the health status of their
herds to reduce antimicrobial use but this is not yet clarified.
Overall, the use of antimicrobials in animals can have an impact on resistance in Man but by far and away the most important contributor to antimicrobial resistance in Man is direct human use.
References
EC. 2011. European Commission—Communication from the Commission to the European Parliament and the Council—Action plan against the rising threats from Antimicrobial Resistance. Swann M.M. 1969. Report by the Joint Committee on the Use of Antibiotics in Animal Husbandry and Veterinary Medicine. SCAN. 1980. Report of the Scientific Committee for Animal Nutrition on the use of Avoparcin in feedingstuffs for Chickens and Pigs (Second Series) Report 6918 EN (CDE-NK-80-002-EN-C). Edmond M.B., Ober J.F., Weinbaum D.L., Pfaller M.A., Hwang T., Sanford M.D. and Wenzel R.P. 1995. Vancomycin-resistant Enterococcus faecium bacteremia: risk factors for infection. Clin Infect Dis 20, 5:1126–1133. Smith T.L., Pearson M.L., Wilcox K.R., Cruz C., Lancaster M.V., Robinson-Dunn B., Tenover F.C., Zervos M.J., Band J.D., White E. and Jarvis W.R. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. Glycopeptide-intermediate Staphylococcus aureus Working group. N Eng J Med 340, 7:493–501. Dobbin K.R. and Dunworth B.A. 1996. Vancomycin-resistant Enterococcus: case reviews after transplantation. AACN Clin Issues 7, 3:403–408. Doores S., Hurd H.S., Hayes D., Mathew A., Maurer J., Silley P., Singer R.S. and Jones B. 2003. Low-level risk assessment for tylosin use in poultry and swine on the treatment of human food-borne disease. Proc In Conf on Antimic Agents and Chemotherapy, Chicago, USA, Abst C2, p1502. Burch D.G.S. 2002. Risk assessment—Campylobacter infection transmission from pigs to man using erythromycin resistance as a marker. Pig Journal 50:53–58. Casewell M., Friis C., Marco E., McMullin P. and Phillips I. 2003. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J Antimicrob Chemother Aug. 52(2):159-161. Danmap 2010. 2011. Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. Voss A. Loeffen F, Bakker J, Klaasen C. and Wulf M. 2005. Methicillin-resistant Staphylococcus aureus in pig farming. Emerg Infect Dis. 11:1965–1966. De Neeling A.J., Van den Broek M.J.M., Spalburg E.C., Van Santen-Verheuvel M.G., Dam-Deisz W.D.C., Boshuizen H.C., Van de Giessen A.W., Van Duijkeren E. and Huijsdens X.W. 2007. High prevalence of methicillin resistant Staphylococcus aureus in pigs. Vet Micro 122:366–372. EFSA. 2009. Scientific report of EFSA. Analysis of the baseline survey on the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU, 2008. Part A: MRSA prevalence estimates. EFSA Journal 7, 11:1376. EFSA. 2010. Scientific report of EFSA. Analysis of the baseline survey on the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU, 2008. Part B: Factors associated with MRSA contamination of holdings. EFSA Journal 8, 6:1597. HPA. 2006. Report Health Protection Agency. Staphylococcus aureus bacteraemia: England, Wales and Northern Ireland, January to December 2005. HPA. 2012. Report Health Protection Agency. Quarterly Epidemiological Commentary: Mandatory MRSA, MSSA and E.coli Bacteraemia and C. difficile Infection Data (up to January–March 2012). Cuny C., Nathaus R., Strommenger B., Altmann D. and Witte W. 2009. Nasal colonization of humans with methicillin-resistant Staphylococcus aureus (MRSA) CC398 with and without exposure to pigs. PLoS ONE, August: e6800. EFSA. 2011. European Food Safety Authority, Panel on Biological Hazards (BIOHAZ)—Scientific Opinion on the public health risks of bacterial strains producing extended-spectrum beta-lactamases and/or AmpC beta-lactamases in food and food-producing animals. EFSA Journal 9, 8:2322. MARAN 2009. 2011. Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands in 2009. VMD. 2010. Veterinary Medicines Directorate Report. Overview of Antimicrobial Usage and Bacterial Resistance in Selected Human and Animal Pathogens in the UK: 2007. EMA. 2011. European Medicines Agency Report. Trend in the sales of veterinary antimicrobial agents in nine European countries. Reporting period 2005–2009. EMA/238630/2011. European Council. 2012. Council of the European Union. Council conclusions on the impact of antimicrobial resistance in the human health sector and in the veterinary sector. A 'One Health' perspective.November 2012






