



Indicator traits and their application to selection for disease resilience
Disease challenges in swine production continue to reduce output and temper profitability potential (VanderWaal and Deen, 2018). Treatments are expensive and vaccines are not fully effective. Moreover, existing pathogens can transform into new variants, and there is a continued risk for new infectious agents (Fournie et al., 2015). New or mutated agents will likely require new or additional forms of treatment and vaccines. It would then seem beneficial for pigs to possess higher resilience to these challenges. Higher resilience would limit the initial impact of disease or would allow a quicker recovery from infection caused by the disease, reducing costly inputs and thus maintain production. As estimated in Knap and Doeshl-Wilson (2020) for countries such as Canada, the United States and in Europe, the costs associated with treating only PRRS-related health challenges equates to more than 4 times the estimated amount of economic value annually realized per pig in genetic improvement. The economic impact of disease is large and introducing measures of resilience into the breeding goal will benefit producer profit.
Disease resilience traits are ‘gold-standard’ phenotypes that reflect the impact from disease (Putz et al., 2019, Cheng et al., 2020). These traits are measured under a disease challenge environment and typically include treatment rate, growth rate, health score, and mortality (did the animal succumb to its challenge). Indicator traits provide information regarding the disease resilience traits but are measured under a high-health or non-challenged environment. For an indicator trait to be beneficial in a breeding program, it must be quantifiable with high accuracy of measurement and have sufficient variation among animals. Indicator traits should also correlate highly with one or more of the disease resilience traits. Measuring indicator traits at the individual pig level provides the necessary base information to derive a genetic selection strategy to improve disease resilience.
One challenge to measuring resilience is acquiring resilience phenotypes on animals that must maintain a clean, high-health status. To accomplish this feat, one option is to continually measure resilience phenotypes in a health challenged setting on close relatives of selection candidates. This approach is expensive and risky, from both an animal welfare standpoint and risking disease transfer to other sites. An alternative approach is to identify indicators of resilience, which are traits themselves that are known to accurately predict resilience trait outcomes under health challenge. Key to this approach, foundation research is initially necessary to discover the link between indicator and resilience phenotypes. This groundwork has begun, and some promising results are reported.
Immune assays can predict health outcomes under disease challenge. These assays measure abundance or function of leukocytes (white blood cells), which are the immune system cells responsible for protection against infection from pathogens. Whole-blood samples are routinely collected and easily obtainable, providing the ability to isolate leukocytes and objectively measure their activity. One such activity is phagocytosis, which is the host cell’s method for engulfing and digesting foreign cells. It is a fundamental process of the body’s immune system, and is the primary method applied by cells to identify and destroy invaders. A phagocytosis assay measures how efficiently the immune cells perform that process (Hampton and Winterbourn, 1999). By analyzing results from this assay, estimates of heritability and genetic correlations will determine if the relationships are strong enough for these indicators to be useful in predicting disease resilience for genetic improvement.
To characterize another immune system process is to focus on the reaction intensity of leukocytes to foreign stimuli. A blood stimulation assay measures cell proliferation, which is how quickly the white blood cells are assembled to fight when challenged with a foreign substance. Jeon and colleagues (2021) recently evaluated and discovered these measurements to be heritable (0.22 – 0.33 when measured 72 hours post-stimulation), as well as favorably genetically correlated with resilience traits in swine. The genetic correlations ranged between -0.40 to -0.60 for finishing mortality, 0.10 to 0.30 for finishing ADG, -0.20 to -0.50 for treatments in nursery, and -0.30 to -0.60 for nursery mortality. A summarized look at the heritability and genetic relationships is outlined in table 2.
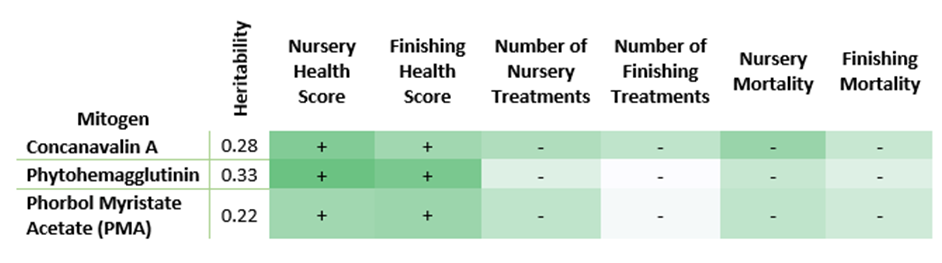
With the knowledge of their favorable genetic correlations, the opportunity to utilize resilience traits by measuring their indicator traits becomes realistic. Furthermore, these indicators can be measured on high-health animals under normal production circumstances. Genesus has invested over a decade of time and research effort in understanding and improving the genetics underlying pig health. Integrating these measurements from nucleus selection candidates into the Genesus genomic breeding program will enable us to select pigs with high genetic merit for disease resilience. Higher resilience equates to lower input costs, better production, lower mortality and ultimately increased profitability for Genesus customers.
| References | ||||
|---|---|---|---|---|
| Cheng J., Putz A.M., Harding J.C.S., Dyck M.K., Fortin F., Plastow G.S., PigGen Canada, Dekkers J.C.M. 2020. Genetic analysis of disease resilience in wean-to-finish pigs from a natural disease challenge model. Journal of Animal Science. 98(8) 1-14. | ||||
| Fournié G., Kearsley-Fleet L., Otte J. 2015. Spatiotemporal trends in the discovery of new swine infectious agents. Veterinary Research 46, 114. | ||||
| Hampton M. B., & Winterbourn C. C. 1999. Methods for quantifying phagocytosis and bacterial killing by human neutrophils. Journal of Immunological Methods, 232(1-2), 15–22. | ||||
| Jeon R.L., Gilbert C., Cheng J., Putz A.M., Dyck M.K., Plastow G.S., Fortin F., Dekkers J.C.M., Harding J.C.S. 2021. Proliferation of peripheral blood mononuclear cells from healthy piglets after mitogen stimulation as indicators of disease resilience. Journal of Animal Science. https://doi.org/10.1093/jas/skab084 | ||||
| Knap, P.W., Doeschl-Wilson, A. 2020. Why breed disease-resilient livestock, and how? Genet Sel Evol 52, 60. | ||||
| Putz AM, Harding JCS, Dyck MK, Fortin F, Plastow GS, Dekkers JCM and PigGen Canada (2019. Novel Resilience Phenotypes Using Feed Intake Data From a Natural Disease Challenge Model in Wean-to-Finish Pigs. Frontiers in Genetics. 9:660 1-14. | ||||
| VanderWaal K. and Deen J. 2018. Global trends in infectious diseases of swine. Proceedings of the National Academy of Sciences. 115(45) 11495-500. | ||||







