



MHYOSPHERE®PCV ID: ESPHM Publications 2021
MHYOSPHERE® PCV ID is the first and only vaccine based on a recombinant Mycoplasma hyopneumoniae (M. hyo) strain, called Nexyhon. This strain expresses the capsid proteins of Porcine Circovirus Type 2 (PCV2) in its cytoplasm and acts as the only active substance as a whole once inactivated. Furthermore, it was the first vaccine registered in Europe with demonstrated protection against all three PCV2 genotypes (a, b and d).This article reviews the different studies that have been conducted with the product to demonstrate its efficacy, both experimentally and in the field, and its beneficial effects on growth until the end of fattening period.
First, the efficacy of the vaccine was evaluated against both M. hyo and PCV2 under experimental conditions. To determine the duration of immunity for each pathogen (M. hyo and PCV2), two separate trials were performed1. In each trial, three-week-old piglets were randomly divided into two groups (vaccinated and control) at the time of vaccination. A single 0.2 ml dose was administered intradermally to vaccinated pigs (MHYOSPHERE® PCV ID) and control pigs (PBS), using Hipradermic® 3.0.
A M. hyo challenge with a highly pathogenic strain was performed intranasally for three consecutive days, 23 weeks after vaccination.
Three weeks after the M. hyo challenge, the pigs were necropsied to assess lung lesions as an efficacy parameter.
For the PCV2 part, as mentioned, a separate study was conducted in which pigs were also vaccinated at three weeks of age and a challenge was performed with intranasal PCV2b, 22 weeks after vaccination.
After this challenge, blood samples were taken from the pigs on a weekly basis to determine viraemia by quantitative PCR (qPCR). Four weeks after the challenge, all the pigs were necropsied and their mesenteric lymph nodes, tonsils, and lungs were taken for quantification of PCV2 by qPCR.
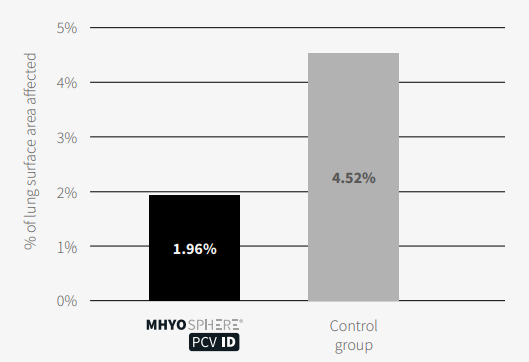
Regarding the results, the lung surface area affected by lesions induced by M. hyo was significantly lower in the group vaccinated with MHYOSPHERE® PCV ID than in the control group (1.96% versus 4.52%, respectively; p <0.05, Welch’s test) (Figure 1).
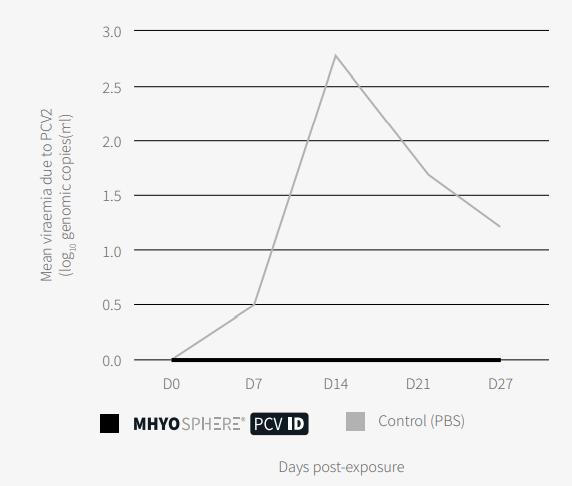
Regarding PCV2, there was no viraemia in the group vaccinated with MHYOSPHERE® PCV ID, as can be seen in Figure 2, and the mean PCV2 viral load was significantly lower (p <0.05, Mann–Whitney U test) in the tonsils, lungs, and mesenteric and inguinal lymph nodes..
Field tests
Once the efficacy of the vaccine had been tested and determined under experimental conditions, the second part of these tests, consisting of field tests, was done.
To demonstrate the efficacy of MHYOSPHERE® PCV ID against M. hyo and PCV2, seven commercial farms with circulation of M. hyo and PCV2 were selected and included in a multicentric, randomised, blinded, negative-control study2. A total of 2,507 healthy three-week-old piglets weredivided into two groups.
One group (n = 1,253) was vaccinated with MHYOSPHERE®PCV ID, while the other group (n = 1,254) received a placebo. A single 0.2 ml dose was administered intradermally to both groups using a needle-free device (Hipradermic®3.0), and the animals were followed up until the end of the fattening period.
The main efficacy endpoints were M. hyo-like lung lesions at slaughter, evaluated in all animals, and the efficacy parameter used for PCV2 monitoring was viraemia, evaluated by qPCR (VetMAXTM PCV2 Quant Kit, Thermo) in 30 animals per group and farm, periodically sampled throughout the study. Genotyping of PCV2 from each farm was done by PCR.
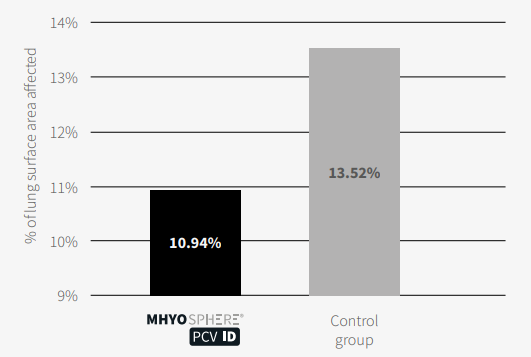
The mean percentage of lung surface area affected by M. hyo-like lesions at slaughter was significantly lower in the group vaccinated with MHYOSPHERE® PCV ID than in the placebo group (10.94% versus 13.52%, respectively; p <0.0001, Mann–Whitney U test), corresponding to a 19.1% reduction.
Furthermore, the incidence of pigs with at least one M. hyo- like lung lesion was also significantly lower in the vaccinated group (p <0.01, X2 test)
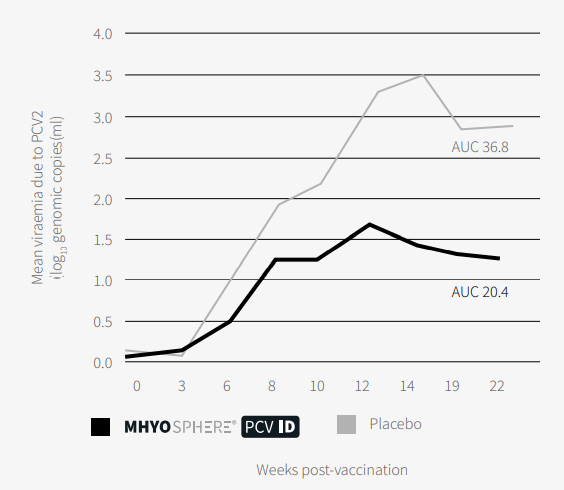
Regarding PCV2, viraemia was significantly lower in the vaccinated group from weaning to the end of the fattening period (Figure 4).
Genotyping results indicated that one farm of seven (14.3%) was PCV2a-positive, three farms (42.8%) were PCV2b-positive and two farms (28.5%) were PCV2d-positive. Only one genotype was found circulating on each farm. In the remaining farm, there was low recirculation of PCV2, and the genotype of the virus could not be determined.
Regardless of the PCV2 genotype identified on each farm, the reduction in viraemia generated by the vaccine was seen to be significantly lower (p <0.001, linear mixed model) in the groups vaccinated with MHYOSPHERE® PCV ID compared to the control group. By genotype, the differences were:
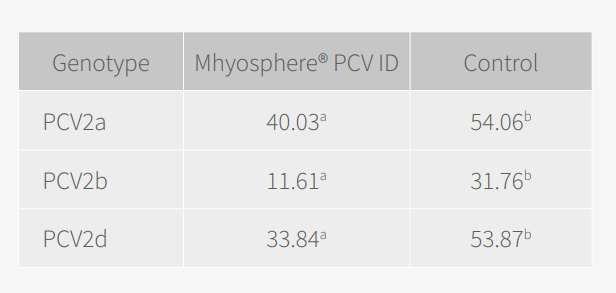
Therefore, based on these results, it was possible to conclude that MHYOSPHERE® PCV ID confers protection against the three most prevalent PCV2 genotypes — a, b and d — as stated in its indications.
Improvements in growth performance under field conditions
To evaluate the effect of MHYOSPHERE® PCV ID on growth, the seven farms mentioned in the previous section and with circulation of M. hyo and PCV2 were used, with a total of 2,507 piglets, 1,253 vaccinated with MHYOSPHERE® PCV ID and 1,254 as a control group (PBS)3.
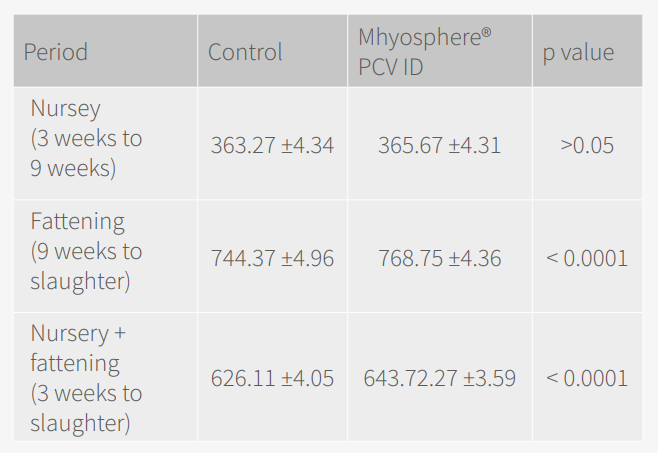
A total of 100 animals/group/farm were weighed at 3 weeks of age, at 9 weeks of age and before slaughter. Growth results were evaluated by average daily gain (ADG), final body weight and culling rate before slaughter (<75 kg).
Regarding the results, the ADG was higher in the MHYOSPHERE® PCV ID group from vaccination (3 weeks of age) to the end of the fattening period (Table 2), with an improvement of 18 g/day (p = 0.0004, linear mixed model taking into account the farm as a random effect).
The nursery results also show the effect of the safety of the vaccine, as piglet yield was not compromised by general reactions, which can negatively impact the growth of these animals in this critical phase.
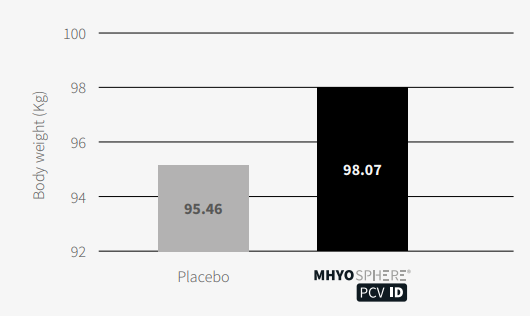
Therefore, at the end of the fattening period, the mean body weight in the MHYOSPHERE® PCV ID group was significantly higher (Figure 5) than in the control group with a difference at the end of the fattening period of 2.61 kg (p<0.001, linear mixed model taking into account the farm as a random effect).
Statistically significant differences were also detected in the culling rate just before slaughter with 5.2% fewer pigs weighting less than 75 kg at slaughter in the MHYOSPHERE® PCV ID group (p <0.001, X2 test).
Take home messages:
MHYOSPHERE® PCV ID proved to:
- Reduce pulmonary lesions associated with enzootic pneumonia of swine caused by Mycoplasma hyopneumoniae. Also reduce the incidence of these lesions (as observed in field studies).
- Reduce PCV2 viraemia and the duration thereof, and reduce the tissue viral load in the tonsils, lungs, and mesenteric lymph nodes.
- Provide cross-protection among different PCV2 genotypes (a, b and d), as also seen in field studies.
- Have direct implications for growth, showing a higher ADG between weaning and before slaughter, and a lower culling rate (<75 kg) at the end of fattening.
| References | ||||
|---|---|---|---|---|
| 1. Montané, J.; Simon-Grifé, M.; Moros, A.; Puigvert, E.; González-González, L.; Pedernera, C.; Acal, L.; Sitjà, M.: Duration of immunity of a novel intradermal vaccine against Mycoplasma hyopneumoniae and PCV2 infection, ESPHM 2021, IMM-PP-25. | ||||
| 2. Montané, J.; Simon-Grifé, M.; Moros, A.; Puigvert, E.; González-González, L.; Pedernera, C.; Acal, L.; Sitjà, M.: Efficacy of a new intradermal vaccine against Mycoplasmahyopneumoniae and PCV2 infection. ESPHM 2021, IMMPP-29 | ||||
| 3. Puig, A.; Perozo, E.; Sabaté, D.; Moros, A.; Roura, F.; March, R.; Montané, J.; Effect of Mhyosphere® PCV ID on the gowth performance under field conditions, ESPHM 2021, IMM-PP-20. | ||||







