



PRRS PCR and PRRS ELISA Responses in Feeder Pigs after Vaccination with Fostera PRRS
A field study was undertaken in order to examine post-vaccination responses to Fostera PRRS™ as assessed by PCR and ELISA, comparing responses in pigs mass-vaccinated by a vaccination crew to those of pigs individually vaccinated, reports Zoetis.Porcine reproductive and respiratory syndrome virus (PRRSV) is widely recognized as one of the most significant economic threats to profitable pork production, estimated to exert a cumulative impact of more than $1 billion per year for US swine producers.1 Since more than half of weaning- age PRRSV-negative pigs become infected with PRRSV before going to market,1 vaccination and other control measures are required to help growing pigs defend themselves against PRRSV.
Diagnostic tools used to monitor PRRSV exposure to field virus and vaccination responses often include two methods. Polymerase chain reaction (PCR) is used to detect virus in blood samples, while enzyme-linked immunosorbent assay (ELISA) reveals the presence or development of circulating antibodies in the blood as an immunological response to viral exposure.
A field study was undertaken in order to examine post-vaccination responses to Fostera PRRS™ as assessed by PCR and ELISA, comparing responses in pigs mass-vaccinated by a vaccination crew to those of pigs individually vaccinated.2
Fostera™ PRRS
Fostera PRRS, from Zoetis, is the first and only PRRSV vaccine that helps prevent respiratory disease associated with PRRSV with a duration of immunity of at least 24 weeks. Fostera PRRS helps optimize performance by helping minimize the adverse affects of a subsequent PRRSV challenge, thereby allowing growing pigs to help maximize their post-challenge weight gain.3 Fostera PRRS offers a single-dose, easy-to-use formulation containing a modified-live PRRSV created from an attenuated US field strain.
Fostera PRRS is approved for the vaccination of healthy, susceptible swine three weeks of age or older in PRRSV-positive herds or sero-negative pigs deemed at risk for exposure to PRRSV as an aid in preventing respiratory disease associated with PRRSV. Fostera PRRS is administered as a single 2-mL intramuscular (IM) dose, after aseptic rehydration of the freeze-dried vaccine with sterile diluent provided.
Experiment Design
The non-clinical vaccine-response study involved 5000 feeder pigs approximately eight weeks of age that were sourced from a PRRS-naive sow farm and placed at a commercial finishing operation in Iowa. Pigs were stocked in 40 pens at the rate of 125 per pen. After one week in the finisher, most pigs (exceptions follow) were vaccinated with Fostera PRRS on study day 0 (Figure 1). The vaccine was administered by a vaccination crew comprised of two adults, two high-school students, and a 12-year-old. Pigs were crowded in each pen and mass-vaccinated IM using Prima Tech hose-draw syringes.
Figure 1. Study design and timeline.
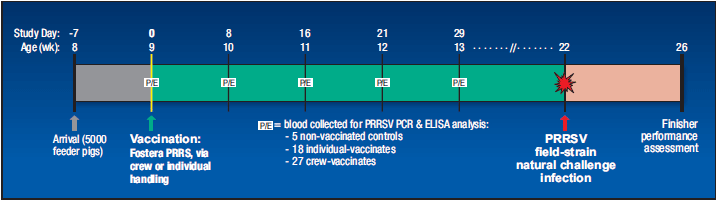
For monitoring of vaccine responses, 10 pigs in each of five separate pens (total of 50 pigs) were randomly selected and tagged for blood sampling during the study. These 50 pigs were managed as follows:
- Controls: one pig in each of the five pens remained non-vaccinated (n=5)
- Individual vaccination: nine pigs in each of two pens (n=18) were individually snared and vaccinated IM with 2mL of Fostera PRRS on day 0 using a disposable syringe with a 16-gauge, 5/8-inch needle;
- Crew vaccination: nine pigs in each ofthree pens (n=27) were vaccinated with Fostera PRRS on day 0 by the vaccination crew, just like all other pigs populating the facility.
Blood samples were collected from designated pigs on study days 0, 8, 16, 21 and 29 and analyzed for PRRSV status using PCR and ELISA. The same 50 pigs were used for each sample date.
Whole-herd data relating to average daily gain (ADG), feed efficiency (feed/gain), mortality rate, and marketing parameters were collected through the entire finishing period. Outcomes were compared to data collected during the previous grow-finish cycle in the same barn involving feeder pigs from the same source; however, the earlier population did not receive PRRS vaccination. During both production cycles, pigs experienced a natural challenge with PRRS field virus at approximately week 14 of the finishing period (sequence similarity of 91.4 per cent for the infecting PRRSV strain involved in each episode).
Because of the non-clinical nature of this demonstration study, no statistical analyses were performed on collected data. Rather, results were generated to satisfy the curiosity of the facility veterinarian and producer, and to exploit an opportunity to learn more about vaccination responses that occur under ‘real-world’ crew vaccination situations.
Results
PCR and ELISA
All sampled pigs were PCR- and ELISA-negative for PRRSV on day 0 of the study (day of vaccination).
PCR results summarized in Table 1 reveal that 100 per cent of the individually vaccinated pigs were PCR positive by eight days following vaccination compared to 96 per cent of the crew-vaccinated pigs. Though not a quantitative assessment, cycle threshold (Ct) values suggest that the amount of vaccine virus in individual-vaccinates appeared to be greater than the amount of virus in crew-vaccinates. Non-vaccinated control animals were 100 per cent PCR-positive on day 21, suggesting that the modified-live vaccine virus was eventually transmitted to non-vaccinated pen-mates.
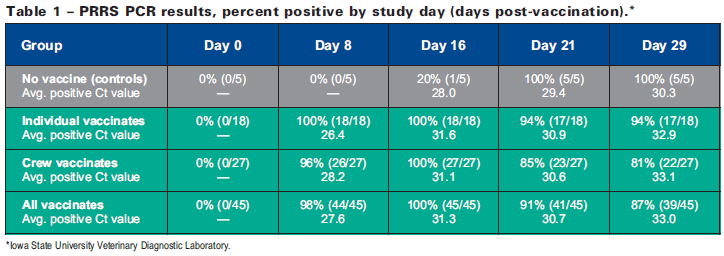
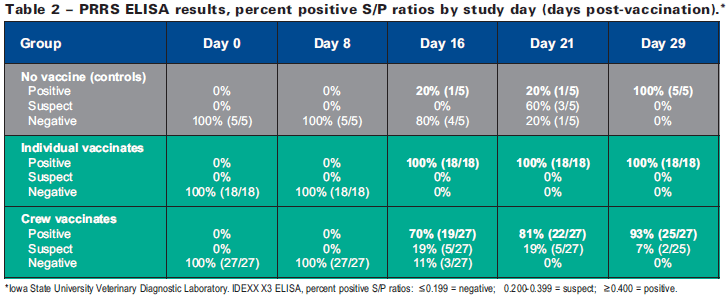
Detection of PRRSV circulating antibodies using ELISA typically lags PCR virus detection because of the time required for antibody production. ELISA results summarized in Table 2 show that 100 per cent of individually vaccinated pigs were ELISA positive by day 16 following vaccination compared to only 70 per cent of crew-vaccinated pigs.
At day 21, 81 per cent of crew-vaccinates were fully ELISA positive, but the response rate rose to 93 per cent by day 29 and the remaining two sampled animals were within the ELISA ‘suspect’ range and expected to subsequently sero-convert. For non-vaccinated controls, seroconversion remained low until day 29 when 100 per cent seroconversion was confirmed, again providing evidence that the vaccine virus was transmitted to non-vaccinated pen-mates over time.
Finisher performance
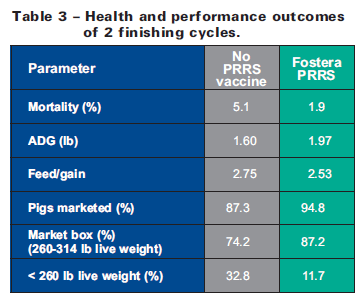
Results presented in Table 3 indicate that mortality was reduced and average daily gain (ADG), feed/gain, and marketing parameters were improved during the study finishing period that included administration of Fostera PRRS vaccine compared to the previous production cycle involving non-vaccinated pigs that were ill-equipped to combat the natural PRRSV challenge.
The producer and veterinarian attributed the following production benefits to Fostera PRRS vaccination (relative improvements versus the previous non-vaccinated herd):
- 63 per cent reduction in death loss
- 19 per cent improvement in ADG
- 8 per cent improvement in feed/gain (dietary fat inclusion also likely involved)
- 8 per cent improvement in pigs marketed
- 15 per cent improvement in pigs ‘in the box’ (260-314 lb);
- 64 per cent reduction in pigs <260 lb.
These outcomes demonstrate that Fostera PRRS helped finishing pigs at this facility perform optimally in the face of a natural PRRSV challenge, even when disease exposure occurred later in life.
The Fostera PRRS duration of immunity appeared sufficient to help generate protective benefits that resulted in an estimated 10:1 return-on-investment at this commercial operation.
Discussion
The PCR and ELISA results observed in this study appear to demonstrate a difference in the rate of effective immunization achieved by typical mass vaccination utilizing a vaccination crew compared to ‘ideal’ vaccination procedures more carefully performed on an individual-pig basis (such as for vaccine clinical trials).
In this study, crew-vaccinates clearly experienced a delay in achieving 100 per cent PCR- and ELISA-positive results. Possible explanations for such observations might include decreased vaccine dosage (e.g. syringe setting too low), failure to effectively administer a full dose to individual pigs, or missed pigs as the crew rapidly moves through the barn. As a result, the development of virus neutralizing antibodies (which typically lags behind antibodies detected via PRRS ELISA testing and are critical for ultimate viral clearance from the pig) could potentially be delayed, thus reducing PRRS protection and expanding the period of disease vulnerability.
Failure to effectively administer a full dose of vaccine to pigs probably accounts for the slower/ lower development of antibodies or PCR-positive rates in commercial herds. However, transmission of vaccine virus was demonstrated in this study by the observation that non-vaccinated controls became PCR- and ELISA-positive, though responses developed considerably later than vaccinated pen-mates. These results suggest that pigs accidentally missed during mass-vaccination may eventually acquire PRRS protection but must first endure a slightly longer period of disease risk.
Conclusions
Proper administration technique is an important component of vaccine protocols.
This non-clinical demonstration study illuminated differences in effective immunization achieved by typical mass vaccination compared to more careful individual vaccination, evidenced by delayed PCR and ELISA responses in crew-vaccinated pigs.
In addition, performance of the study herd was dramatically enhanced when pigs were vaccinated with Fostera PRRS prior to natural PRRSV challenge compared to non-vaccinated pigs comprising the previous production cycle.
References
1. Holtkamp D, Kliebenstein J, Zimmerman J, et al. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on US pork producer. National Pork Board Research Report 2011; NPB #10-158.
2. Data on file, Study Report No. 12 PETBIO01, Zoetis Inc.
3. Data on file, Study Report No. 3127R-60-10-890, Zoetis Inc.
August 2013







