Syringeability of Hyogen®, a Vaccine Against Mycoplasma hyopneumoniae
Pneumonia induced by M. hyopneumoniae is considered one of the most wide spread and most important chronic diseases in pigs, write P. Forget, S. Lacoste, S. Gobbi, P. Mazerolles, R. Krejci and A. Lopez, Ceva.Over 70% of pig herds are vaccinated in countries with high developed swine industry. Mass vaccination is highly efficient in the protection against the development of bronchopneumonia but it is also demanding for the labor considering huge number animals to be injected. In this respect the easiness of the vaccine administration is important together of course with the safety and efficacy of the product.
Hyogen® (Ceva) is an injectable inactivated M. hyo vaccine, proven efficient in preventing the lung
lesions induced by M. hyo infection. The aim of the study was to assess the syringeability of Hyogen®
in comparison with selected major competitor vaccines.
Materials and methods
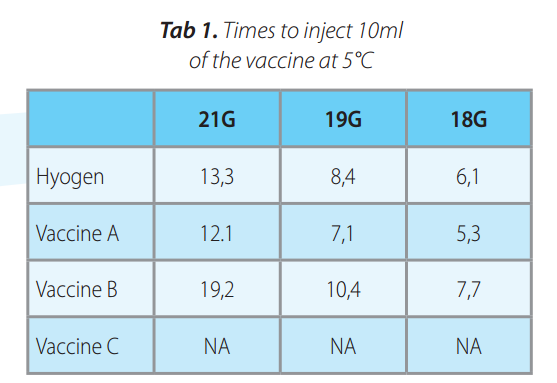
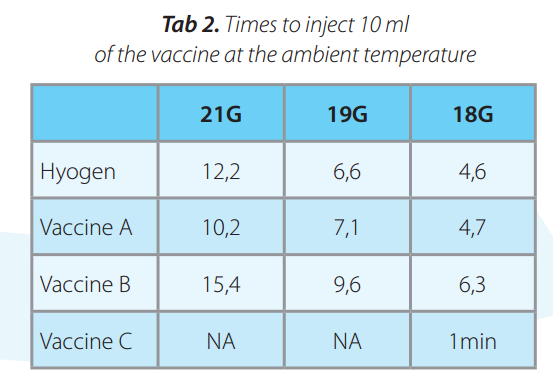
Hyogen® containing Imuvant T M a s a n adjuvant was compared with three commercial vaccines: Vaccine A containing Amphigen, vaccine B containing carbopol and vaccine C containing Impran as adjuvants. All products were measured at 5°C and ambient temperatures for syringeability in 21G, 19G and 18G needles. In all condition the glass syringe was filled in with the product and the time needed for injecting 10 ml volume under the constant pressure of 10N was measured in six replicates. The mean values were compared.
Results
At 5°C the Vaccine C was blocked in the needles (not injectable) irrespectively of the diameter. Hyogen® and vaccine A had shorter cumulative mean time than Vaccine B or Vaccine C (p<0.05).
At ambient t° Hyogen® and Vaccine A had shorter cummulative mean time than Vaccine B or Vaccine C (p<0.05). Time required for Vaccine C exceeded 1min, 3min and 7min in 18G, 19G and 21G needles respectively.
Conclusions
Hyogen® together with Vaccine A proved to be best injectable M.hyo vaccine of four different
commercial products tested. Vaccines B and C (with carbopol and Impran) required significantly longer time at both 5°C or ambient temperatures.