



University of Maryland performs 2nd pig heart transplant
Patient with end-stage cardiovascular disease received gene-edited pig heartAfter world’s first successful transplant in 2022, also performed at the University of Maryland Medical Center (UMMC), this groundbreaking transplant team performed second pig heart transplant on patient deemed ineligible for traditional heart transplant.
A 58-year-old patient with terminal heart disease became the second patient in the world to receive a historic transplant of a genetically-modified pig heart on September 20. He is recovering and communicating with his loved ones. According to reports, he is said to be stable and doing well after the operation, and the organ passed its first test by avoiding hyperacute rejection.
This is only the second time in the world that a genetically modified pig heart has been transplanted into a living patient. Both surgeries were performed by University of Maryland School of Medicine (UMSOM) faculty at the University of Maryland Medical Center (UMMC).
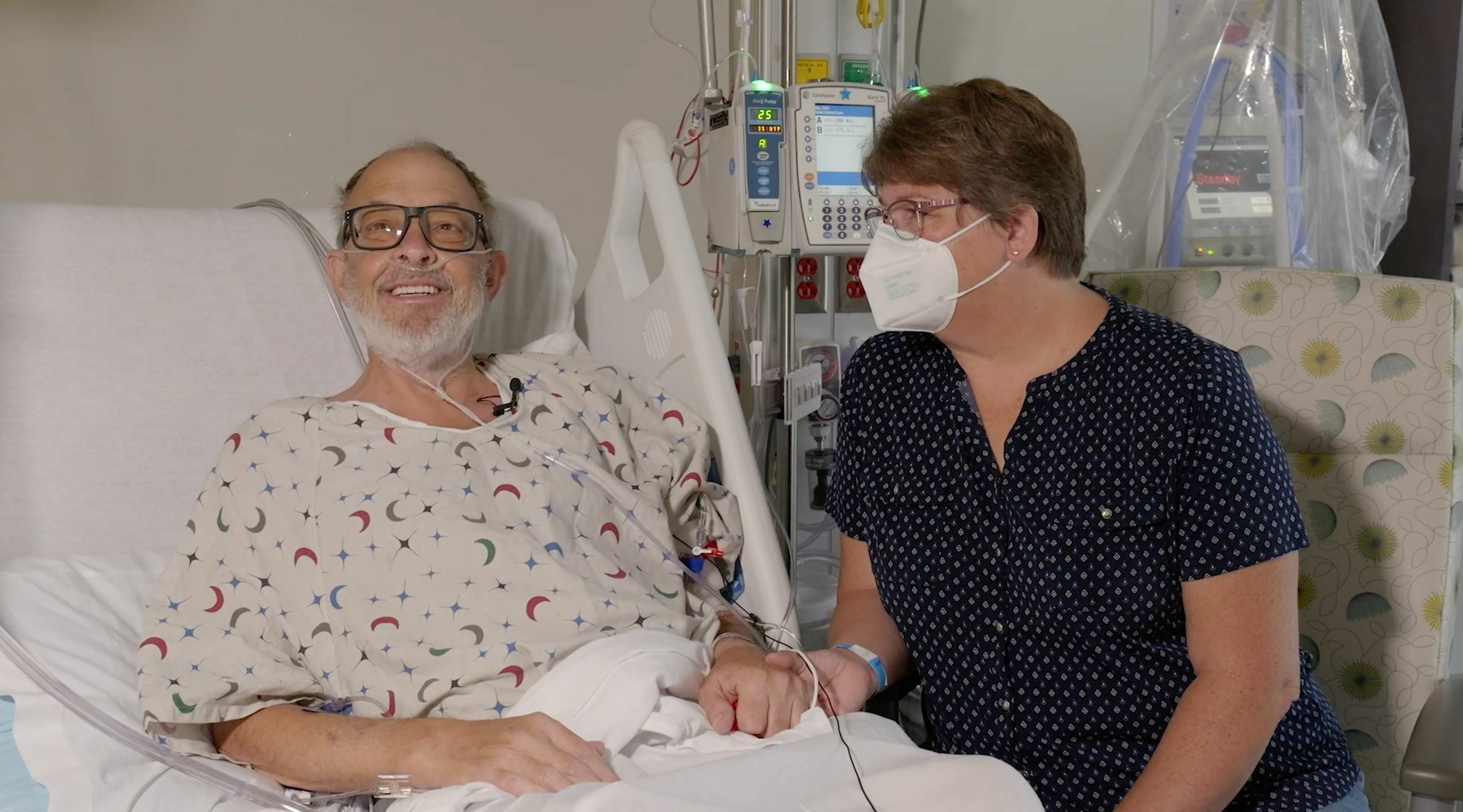
The first historic surgery, performed in January, 2022, was conducted on David Bennett by University of Maryland Medicine surgeons (comprising UMSOM and UMMC), who are recognized as the leaders in cardiac xenotransplantation. This new patient, Lawrence Faucette, had end-stage heart disease. He was deemed ineligible for a traditional transplant with a human heart, by UMMC and several other leading transplant hospitals, due to his pre-existing peripheral vascular disease and complications with internal bleeding.
This transplant was the only option available for Mr. Faucette who was facing near-certain death from heart failure. The patient, who lives in Frederick, MD, is a married father of two and a 20-year Navy veteran and most recently worked as a lab technician at the National Institutes of Health before his retirement. He is currently breathing on his own, and his heart is functioning well without any assistance from supportive devices.
“My only real hope left is to go with the pig heart, the xenotransplant,” said Mr. Faucette during an interview from his hospital room a few days before his surgery. “Dr. Griffith, Dr. Mohiuddin and their entire staff have been incredible, but nobody knows from this point forward. At least now I have hope, and I have a chance."
Added his wife, Ann Faucette: “We have no expectations other than hoping for more time together. That could be as simple as sitting on the front porch and having coffee together."
Gene-edited pig heart used in
Organs from genetically modified pigs have been the focus of much of the research in xenotransplantation, in part because of physiologic similarities between pigs and human and nonhuman primates. United Therapeutics has funded a $22 million research program to test their genetically-modified pig hearts from Revivicor in baboon studies conducted at UMSOM.
Three genes — responsible for a rapid antibody-mediated rejection of pig organs by humans — were “knocked out” in the donor pig. Six human genes responsible for immune acceptance of the pig heart were inserted into the genome. One additional gene in the pig was knocked out to prevent excessive growth of the pig heart tissue, for a total of 10 unique gene edits made in the donor pig.
United Therapeutics Corporation, through its xenotransplantation subsidiary Revivicor, based in Blacksburg, VA, provided the genetically-modified pig to the xenotransplantation laboratory at UMSOM. On the morning of the transplant surgery, the surgical team, led by Dr. Griffith and Dr. Mohiuddin, removed the pig’s heart and placed it in the XVIVO Heart Box, a machine perfusion device, to keep the heart preserved until surgery.
“This procedure is another significant step forward in bringing our vision of lifesaving xenotransplantation to those patients in desperate need,” said David Ayares, PhD, President and Chief Scientific Officer of United Therapeutics Corporation’s Revivicor subsidiary. “This second successful transplantation of United Therapeutics’ UHeart™ is a product of decades of gene editing, animal husbandry and creative thinking by the team of scientists at United Therapeutics and Revivicor, and at the University of Maryland—especially Drs. Mohiuddin and Griffith. All of us at United Therapeutics recognize the bravery and unconditional willingness by Mr. Faucette to advance the cause of science and medical treatment in this remarkable way.”
Saving the patient's life using xenotransplantation

The U.S. Food and Drug Administration granted emergency approval for the surgery on Friday September 15 through its single patient investigational new drug (IND) “compassionate use” pathway. This approval process is used when an experimental medical product, in this case the genetically-modified pig’s heart, is the only option available for a patient faced with a serious or life-threatening medical condition. The approval was granted in the hope of saving the patient’s life.
“We are once again offering a dying patient a shot at a longer life, and we are incredibly grateful to Mr. Faucette for his bravery and willingness to help advance our knowledge of this field,” said Bartley P. Griffith, MD, who surgically transplanted the pig heart into both the first and second patient at UMMC. Dr. Griffith is the Thomas E. and Alice Marie Hales Distinguished Professor in Transplant Surgery and Clinical Director of the Cardiac Xenotransplantation Program at UMSOM. “We are hopeful that he will get home soon to enjoy more time with his wife and the rest of his loving family.”
Considered one of the world’s foremost experts on xenotransplantation, Muhammad M. Mohiuddin, MD, Professor of Surgery at UMSOM, joined the UMSOM faculty seven years ago and established the Cardiac Xenotransplantation Program. Dr. Mohiuddin serves as the program’s Program/Scientific Director. Dr Mohiuddin co-led this procedure with Dr Griffith.

“We are continuing to pursue the pathway to clinical trials by providing important new data on pre-clinical research that has been requested by the FDA,” said Dr. Mohiuddin. “The FDA used our data from these new studies, as well as our experience with the first patient, to determine that we were ready to attempt a second transplant in an end-stage heart disease patient who had no other treatment options.”
About 110,000 Americans are currently waiting for an organ transplant, and more than 6,000 patients die each year before getting one, according to the federal government’s organdonor.gov. Transplanting animal organs (known as xenotransplantation) could potentially save thousands of lives but carries a unique set of risks. Besides the fear of transmitting an unknown pathogen from the animal to human, xenotransplants are more likely to trigger a dangerous immune response. These responses can trigger an immediate rejection of the organ with a potentially deadly outcome to the patient.
The physician-scientists are also treating the patient with a novel antibody therapy along with conventional anti-rejection drugs, which are designed to suppress the immune system and prevent the body from damaging or rejecting the foreign organ. The novel therapy being developed by Eledon Pharmaceuticals is an experimental antibody, called tegoprubart; it blocks CD154, a protein involved in immune system activation.
Before consenting to receive the transplant, Mr. Faucette was fully informed of the procedure’s risks, and that the procedure was experimental with unknown risks and benefits. He was admitted to UMMC on Thursday, September 14 after experiencing complications from his heart failure and peripheral vascular disease. Mr. Faucette underwent a psychiatric evaluation and met with a medical ethicist, social workers and other members of the UMMC care team to discuss the procedure’s risks and benefits and to obtain his informed consent.
During the nearly two years since the first surgery, UMSOM faculty-scientists have extensively investigated Mr. Bennett’s experience with the world’s first genetically modified cardiac xenotransplant. They published their initial findings in the New England Journal of Medicine and then published their follow-up findings from an extensive investigation in The Lancet. They demonstrated that the pig heart functioned well in the patient for several weeks with no signs of acute rejection. Mr. Bennett’s death from heart failure was likely caused by a multitude of factors including his poor state of health that left him hospitalized on a heart-lung bypass machine for six weeks prior to the transplant.
Prior to performing the first surgery in Mr. Bennett in 2022, Dr. Mohiuddin, Dr. Griffith, and their research team spent five years perfecting the surgical technique on non-human primates. Dr. Mohiuddin’s xenotransplant research experience spans over 30 years, during which time he demonstrated in peer-reviewed research that a genetically-modified pig’s heart can function when placed in the abdomen for as long as three years.






