



Effects of Disease on Pig Performance – A Review
By Pete Bown BVMS FRCVS and Ann Davis BVMS MRCVS, George Veterinary Group, Malmesbury, Wiltshire.Effects of Disease on Pig Performance – A Review
bY Pete Bown BVMS FRCVS and Ann Davis BVMS MRCVS, George Veterinary Group, Malmesbury, Wiltshire.
Summary
Pig performance from weaning to slaughter did not improve throughout the 1990’s. Primarily this was due to the introduction of PRRS virus and Swine influenza virus into the United Kingdom pig populations. Before the industry could recover, PMWS and epidemic PDNS arrived in 1999 and spread throughout the country, causing performance to deteriorate further. The effects of the disease on daily liveweight gain, food conversion efficiency, mortality and drug usage are discussed and where possible quantified. Possible remedial measures are identified.
Introduction
Health and nutrition are the two major factors governing physical and economic performance of pigs from weaning to slaughter. With good nutrition and in the absence of major disease good daily liveweight gain (DLWG) is achieved with improved feed conversion efficiency (FCR).
Prior to 1990 establishing and maintaining a high health status herd was relatively straightforward and maintenance of this high health status was achieved with good biosecurity. At that time specific diseases were controlled by use of antibiotics and vaccines where appropriate and where available.
The introduction of Porcine Respiratory and Reproductive Syndrome (PRRS) virus into the UK in 1991 (White 1991 & Paton et al 1991) presented the British pig industry with a new problem. Antibiotics were of little use and no vaccine was available. New management techniques were adopted to control this serious viral disease.
All-in, allout strategies coupled with three-site production and early weaning, with or without medication, became commonplace and afforded reasonable control of the disease. The health status of the UK pig population stabilised and was not seriously disturbed again until 1999 when Post Weaning Multi-systemic Wasting Syndrome (PMWS) was introduced into England (Potter 2000).
This disease syndrome has had a prolonged and devastating impact on both the physical and economic performance and from which the industry has not yet recovered. The outbreak of Classical Swine Fever in East Anglia in 2000 and of Foot and Mouth Disease in 2001 has added fuel to the fire and left the future viability of the British Pig Industry balancing on a knife edge.
Physiological effects of disease on performance
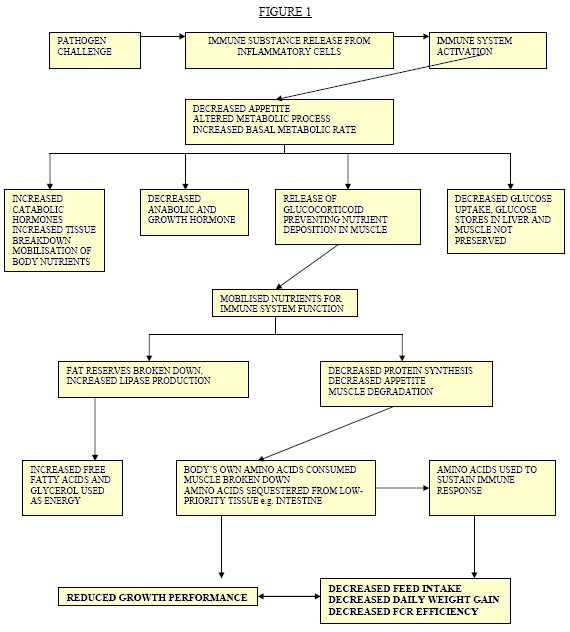
The presence of disease is associated with either weight loss or lowered rate of weight gain depending on severity plus reduced feed conversion efficiency. Energy and protein required to mount an immune response are directed away from muscle growth (Spurlock 1997, Dial 2002). The challenge to the immune system will induce a number of effects: -
- Immunological challenge will cause a decrease in insulin–like growth factor (Hevener 1999). This factor is closely associated with animal growth rates and ultimate body size.
- Cytokines produced as part of the immune response suppress secretion of growth promoting hormones (Klasing 1988).
- Decreased feed intake or anorexia due to pathogenic infection and immune system activation
- The metabolic response to infection causes nutrients to be directed away from tissue growth in support of immune function.
- Concurrent subclinical infections will create cumulative effects, which may significantly worsen performance.
These physiological pathways are summarised in Figure 1.
| TABLE 1 - Expression of disease |
| DLWG FCR Mortality Drug cost Uneven pigs |
Whatever the disease, in whatever body system, the physiological effects are the same, varying only in severity. Prior to 1999 the major disease syndromes adversely affecting pig performance from weaning to finish were Respiratory Disease and Enteric Disease with a few miscellaneous conditions contributing on occasion. In the second half of 1999, Postweaning Multisystemic Wasting Syndrome (PMWS) and associated epidemic Porcine Dermatitis and Nephropathy Syndrome (PDNS) were diagnosed for the first time in the United Kingdom (Potter 2000) and have blighted pig performance ever since. Each of these syndromes will be considered and the individual and combined effects on performance assessed. Disease in a finishing herd is expressed by reductions in DLWG, decreased FCR, raised mortality, increased drug cost and uneven growth (Table 1).
Respiratory Disease
There are three compartments to the respiratory tract, namely upper, middle or lower. The upper respiratory tract comprises the nostrils, nasal cavity, sinuses and nasopharynx and disease of these areas is associated with sneezing. The middle compartment includes the larynx, trachea and main bronchi. Disease affecting these areas is associated with coughing. The lower respiratory compartment is the bronchiolar and alveolar region and disease of this area is associated with dyspnoea (Done et al 1994). The major respiratory pathogens commonly affecting growing pigs in the United Kingdom are identified in Table 2.
| Table 2: Respiratory Tract Pathogens of the Pig | |
| Bacteria | Mycoplasma hyopneumoniae Actinobacillus pleuropneumoniae Haemophilus parasuis Pasteurella multocida Streptococcus suis Pasteurella haemolytica Bordetella bronchiseptica |
| Viruses | PRRS Swine Influenza Porcine Circovirus 2 |
| Helminths | Ascarid larval migration |
Prior to 1990 clinical respiratory disease invariably was associated with a single pathogen, which could be identified fairly readily and treated. If the primary agent was viral or a mycoplasma, secondary bacterial infection occurred commonly but could usually be controlled by use of an antibiotic. In the early 1990’s PRRS descended on the pig world and revolutionised the disease situation in the UK pig population, precipitating a new respiratory syndrome, which became known as the Porcine Respiratory Disease Complex (PRDC). PRRS so changed the health environment that it was not long before swine influenza in all its sub-types entered the arena and added to the severity and complexity of porcine respiratory disease, The clinical signs and therefore economic impact of PRDC depends upon a number of factors including nutrition, management, immune status of the population, strains of primary virus(es) and complexity of secondary bacterial invaders. Morbidity is always high and mortality may be high also.
The economic impact of respiratory disease on any individual unit will depend not only on the clinical severity of the disease but also on current cost of production figures. These vary widely from farm to farm. Feed cost per kilogram live weight gain can vary from 24p to 40p per pig.
Mild respiratory disease will result in decreased feed intake, decreased daily live weight gain (DLWG), deteriorating FCR and the necessity to sell pigs at a lower finished weight. A hidden bonus of mild respiratory disease is a reduction in backfat (P2) of the finished carcase. This observation was confirmed when efficient enzootic pneumonia (EP) vaccines became available. Good control of EP by judicious use of vaccine increased P2 measurement by 1 –2 mm per pig initially until diets were reformulated.
For every 10% of lung tissue affected by pneumonia DLWG is reduced by 22 – 37 grammes per day. Studies in Germany reveal a reduction in DLWG of 34 – 50 g/day in pigs with lung lesions compared to pigs with healthy lungs. (Klawitter et al 1988) In Denmark a combination of EP & APP resulted in a growth reduction of 30.3 – 58.8g/day. (Baekbo et al 2002)
It is estimated that clinical PRRS in growing and finishing pigs reduced DLWG by up to l74g/day in the initial insult (Richardson 2004) When PRDC becomes severe mortality rate and cull rate rise significantly and DLWG can be reduced by up to 50%, at least doubling daily financial losses. During the 1990’s the industry and the veterinary profession began to get to grips with this new disease scenario. New management strategies such as medicated early weaning (MEW) segregated early weaning (SEW) and all-in all-out (AIAO) coupled with the new efficient EP vaccines brought some semblance of control to PRDC. However, in 1999 PMWS arrived in the United Kingdom. Although it moved around the country fairly slowly, over the next two years the health of the U.K. pig population deteriorated once more with disastrous economic consequences.
PMWS/PDNS
PDNS was recorded in the UK by Smith et al (1993) and White and Higgins (1993). Between 1993 and 1998 there have averaged nine recorded outbreaks of this condition in the U.K. (Gresham et al 2000) with low morbidity and mortality. The disease was christened sporadic PDNS.
In 1999 PMWS was diagnosed for the first time in England in association with acute PDNS (Potter 2000). PMWS is believed to be associated with porcine circo virus type 2 (PCV-2), (Ellis et al 1998) but the relationship between PMWS and PDNS is still unclear. Many veterinarians working in the field believe that the causal organism for PMWS is still to be identified.
PMWS and associated epidemic PDNS are now widespread throughout the United Kingdom and over the last five years this disease syndrome has devastated the British Pig industry with high mortality, often up to 25%.
Economic losses have frequently exceeded £10 per pig (Done 2002). In severe outbreaks incurred costs have added 15p per kilogram to the cost of production of a finished pig.
The mode(s) of transmission of the causal agent has not been identified and the disease does not appear to stimulate a herd immunity. Control measures revolve around strict attention to hygiene, good nutrition, stable social groups and all-in all-out housing policies. Shortage of labour on many farms has made efficient implementation of these policies difficult and this disease syndrome is one of the major reasons why the British Pig industry is currently selling 17.4 finished pigs per sow per year (Thames Valley Cambac 2004)
Enteric Disease
Prior to PMWS approximately 70% of disease costs could be related to alimentary and respiratory disease (Done 1999). Disease of the alimentary tract can be broadly divided into small intestinal diseases (enteritis) and large bowel disease (Colitis) Small intestinal disease most commonly occurs within 10 days of weaning and is usually associated with presence of toxigenic E. coli. sometimes complicated by Salmonella species infection. The clinical expression of disease and severity of disease depends not only on infection but also on housing, nutrition and hygiene. Up to 50% of all weaned pigs may be affected and mortality levels may reach 10%. Even when diarrhoea does not persist for very long there can be a significant reduction in DLWG and some animals may be permanently stunted due to chronic intestinal scarring and villous atrophy. Optimum feed intakes post-weaning maximise DLWG and feed conversion efficiency (FCR). Severe post-weaning diarrhoea therefore can increase cost of production by between one and two pence per kilogram.
Since the development of PMWS enteritis has become more common in older pigs, frequently associated with salmonella infection. Diarrhoea is a common clinical feature of PMWS but the cost implications have already been discussed. Inflammation of the large bowel is known as colitis whilst inflammation of the terminal part of the small intestine is known as ileitis. Proliferative enteropathy is a specific disease of the ileum, frequently spreading into the colon and caused by an intracellular organism known as Lawsonia intracellularis. An investigation into field cases of porcine colitis on 85 pig units in the United Kingdom (Thomson et al 1998) identified Brachyspira pilosicoli, Yersinia pseudotuberculosis, Lawsonia intracellularis, Salmonella typhimurium and Brachyspira hyodysenteriae as the major pathogens. There was a combination of pure and mixed infections. Despite successful treatment of batches of pigs colitis persisted as a chronic problem on many units with diarrhoea and body weight loss the main clinical features. Ileitis and Colitis tend to develop in pigs ranging between 20 and 40 kilograms with an estimated prevalence of 5 and 15 per cent. Mortality rate varies widely from 1 per cent to 25 per cent (Taylor 1995).
Ileitis cost can vary from £0.92 per pig to £13.87 per pig depending on severity (Lawrence 1999). Colitis associated with Brachyspira species infection is estimated to reduce FCR by between 0.05 and 0.2, increasing time to finish by up to three weeks. Again the presence of PMWS has had an adverse impact on the prevalence of Colitis, possibly aggravated by the compulsory ban on some antibiotic growth promoters in July 1999. Therefore, it is likely that the economic impact of Ileitis and Colitis has increased since 1999 but there is no published work to confirm this other than the estimations of the cost of PMWS highlighted previously.
Miscellaneous Conditions
Sarcoptic Mange
The most significant ectoparasite of the growing and finishing pig in the United Kingdom is Sarcoptes scabiei var.suis (White 1994). The disease has a serious economic effect on growing pigs as well as welfare implications. The disease increased in incidence during the 1990’s with the advent of chronic respiratory disease associated with immunosuppressant viral infection such as PRRS and Swine Influenza.
A survey of slaughter pigs in the United Kingdom in 1990 identified a 70 per cent incidence (McMullin et al 1992). Cargill and Dobson (1979) reported experimental disease as producing a reduction in DLWG and FCR of between 9.2 and 12.5 per cent whilst Gaafer et al (1986) reported that successful control of the disease produced a 5.5 kg improvement in slaughter weight and a 0.1 improvement in FCR. The author’s personal experience confirms the economic importance of this disease.
Intestinal Parasites
Ascaris suum, the pig ascarid, currently constitutes the major economically significant nematode in the United Kingdom (White 1994). As indicated elsewhere in this review Ascaris Suum must be considered in the differential diagnosis of respiratory disease but also causes high levels of liver condemnations at the abattoir and depressed DLWG and FCR in heavily infested herds. Both growth rate and feed efficiency may be depressed by up to 10 per cent.
In view of the highly resistant nature of the ascarid egg and the move to more extensive housing systems with solid floors, infection with this parasite will remain highly significant for the foreseeable future.
Meningitis
Whilst clinical meningitis can be caused by several bacteria and viruses the commonest cause of meningitis in growing pigs in the United Kingdom is Streptococcus suis type 2 ( Done et al 1998). There are 35 known serotypes of Streptococcus suis and many can cause outbreaks of septicaemia or meningitis in growing pigs (Sandford and Higgins 1992; Higgins et al 1995). Infections can occur at any age but most cases are in piglets between 3 and 12 weeks, although pigs of finishing weights may be affected.
Clinical incidence of the disease varies between 1 & 10 per cent with up to 3 per cent mortality. SEW and MEW technologies do not eliminate Streptococcus suis infection in weaned pigs because pigs are infected during birth or the first few hours of life. (Robertson and Blackmore 1989) and because prophylactic use of antibiotic does not eliminate the carrier state (Amass et al 1996). Consequently Streptococcus suis is responsible for a significant portion of the treatment costs associated with raising high health status pigs.
Arthritis
Arthritis is common in the growing pig. The most common infections of joints are erysipelas, Mycoplasma hyosynoviae and Streptococcus suis. Osteochondrosis (OCD) is the most common non-infectious cause of arthritis. Clinical incidence ranges from 1 to 5 per cent normally, but in outbreaks of erysipelas or Streptococcus suis incidence may rise to 10 per cent. In such circumstances arthritis becomes an economically significant disease resulting in an increased culling rate, increased treatment costs and increased condemnation rate at the abattoir.
Discussion
As with all other types of farming pigs are farmed for profit, hopefully. Profit is derived from the margin between the price received for the finished carcase and the costs incurred to produce that carcase. The major cost is feed and up to 75 percent of total feed purchased is used from weaning to slaughter. Presence of disease adversely affects feed intakes and efficient utilisation of feed. The physiological pathways responsible for this are highlighted in Figure 1. The pathogen load a unit carries is initially related to the health status of the purchased breeding stock, and subsequently is affected by the proximity of other pig farms, unit biosecurity, stockmanship, housing, hygiene and the production system on the farm.
Even before PMWS/PDNS respiratory and enteric disease could reduce unit performance by up to 50 per cent of the genetic potential of the animal (Kingston 1999). During the period 1990 – 1998 there was no improvement in feeding herd performance in terms of DLWG and FCR and over the same period mortality rose (Easicare Yearbook 1990 – 1998). Since that time PMWS/PDNS has appeared and physical performance has deteriorated still further.
The emergence of PRRS and Swine influenza viruses in the 1990’s made the UK pig population more susceptible to the other infectious diseases identified in this paper. The subsequent emergence of PMWS and epidemic PDNS in 1999 has made the situation much worse and the U.K. pig industry has not yet recovered. In addition enteric disease incidence was aggravated by the ban on certain growth promoters in July 1999 and doubtless will be aggravated still further by a total ban on these products in 2006. During the 1980’s and 1990’s use of growth promoters gave an increased income of £2.50 per pig (Lawrence 1998). The compulsory reduction in copper inclusions in pig feeds in 2004 has resulted in increased incidence of loose faeces and the authors have recorded an average reduction in DLWG of 34 grammes per day with a maximum recorded deterioration of 70 grammes per day. The economic impact of disease on any individual unit will depend not only on the severity of the disease but also on current cost of production figures. A 0.1 deterioration in FCR costs between 75 pence and 90 pence (BOCM Pauls Ltd 2004) whilst a reduction of DLWG of 20 grammes per day, costs between £1.00 and £1.50 per finished pig.
To combat the costs of general background pathogen challenge and PMWS many farmers have adopted all-in all-out stocking policies coupled with strict hygiene and disinfection procedures. All have experienced some benefit but on those farms where the individual buildings were close together improvement has been modest, barely compensating for increased costs incurred. In the authors experience the most cost effective management change has been a partial depopulation of the unit with thorough cleansing and disinfection, weaning off site for a period of 4 – 6 weeks with a breeding herd medication programme during that time specifically designed to eliminate pathogens endemic to the unit. Such programmes have reduced time to slaughter by two weeks and given a cost of production benefit of £7.50 - £10.00 per pig. Two years after completion of the programme many producers still have empty finishing pens due to improved physical performance.
These programmes not only demonstrate the benefits of health they highlight, very clearly, the true cost of disease.
ABOUT THIS PAPER
The NATWEST/RAC ANNUAL FELLOWSHIP in STRATEGIC DEVELOPMENT OF THE UK PIG INDUSTRY - 2nd November 2004
A paper presented by Pete Bown BVMS FRCVS & Ann Davis BVMS MRCVS George Veterinary Group, Malmesbury, Wiltshire.
In Support of Dr Keith Lawrence’s Award under the heading of : To challenge the future approach to managing disease in the UK in an ever changing marketplace
REFERENCES
Amass, S.F., Wu, C.C. and Clark, L.K. (1996). Journal of Veterinary Diagnostic Investigation. 8, 64.
Baekbo, P., Andreason , M., Wachmann, H., Christensen, G (2002) Proc. 17th IPVS Congress 103.
BOCM Pauls Ltd. (2004) Personal communication.
Cargill, C.F. and Dobson, K.J. (1979) Veterinary Record 104, 33. Dial, G (2002) London Swine Conference.
Done, S.H.; Brown, I., Paton, D., Higgins, R. and Hannam, D. (1994). The Pig Journal. 33, 133.
Done, S. H., Spencer, Y. I. and Jones, M.A. (1998). The Pig Journal. 41, 113.
Done, S. H. (1999). The Pig Journal. 44, 183.
Done, S. H. (2002). Proceedings of the 17th IPVS, Ames, Iowa 74.
Easicare Yearbook 1990 – 1998.
Ellis, J., Hassard, L., Clark, E. (1998). Canadian Veterinary Journal. 39, 44.
Gaafer, S. M., Arends, J. J., Hogg, A., Holscher, K. M. and Williams, R. E. (1986). Journal Agric. Entomal. 3. 374.
Gresham, A., Jackson, G., Giles, N., Allan, G., McNeilly, F. and Kennedy, S. (2002) The Veterinary Record. 146, 143.
Hevener, W. (1999). Canadian Veterinary Journal. 40, 782.
Higgins, R., Gottschalk, M., Boudreau, M., Lebrum, A. and Henrichsen, J. (1995)
Journal of Veterinary Diagnostic Investigation. 7, 405.
Kingston, N. G. (1999) The Pig Journal 44, 84.
Klasing, K. C., (1998). Journal of Nutrition. 124, 906.
Klawitter, E., Hoy, S., Mehlhorn, G. (1998) Monthly Journal of Veterinary Medicine 43, 597
Lawrence, K. (1998). The Pig Journal. 41, 75.
Lawrence, K. (1999). Proceedings PIC/Elanco Pig Veterinary Conference.
McMullin, P. F., Guise, J., Cuthbertson, C. and Jones, M. A. (1992).
Momentum Vol. 2 (MSD Ag Vet)
Paton, D. J., Brown, I. H., Edwards, S., Wensvoort, G. (1991). The Veterinary Record 128, 617.
Potter, R. A. (2000). The Veterinary Record. 146, 84.
Richardson, J. S. (2004) The Pig Journal 53 176
Robertson, I. D. and Blackmore, D. K. (1989). The Veterinary Record. 124, 391.
Sandford, S. E., Higggins, R. (1992). Diseases of swine 7th Edition 588.
Smith, W., Thomson, J. R. and Done, S. H. (1993). The Veterinary Record. 132, 47.
Spurlock, M. E. (1997) Journal of Animal Science. 75, 1773.
Taylor, D. J. (1995) Pig Diseases 6th Edition 143.
Thames Valley Cambac (2004) Personal communication.
Thomson, J. R., Smith, W. J. and Murray, B. P. (1998). The Veterinary Record. 142, 235.
White, M. E. C. (1991). The Veterinary Record. 128, 574.
White, M. E. C., and Higgins, R. J. (1993). The Veterinary Record. 132, 199
White, M. E. C. (1994). The Pig Journal 33, 41.






