



Field efficacy study of Rhiniseng® in Europe in a farm with problems associated with Bordetella bronchiseptica
Immunisation of pregnant sows with Rhiniseng® prior to farrowing induced a high concentration of serum antibodies, which were efficiently transferred to piglets by colostrum.
Introduction
The metaphylactic use of antimicrobials in pig production in the EU and US has been limited by legislation [1,2], giving way to the development of alternative methods for the prevention of relevant swine diseases like the non-progressive atrophic rhinitis (NPAR) caused by Bordetella bronchiseptica (Bb) [3] in absence of Pasteurella multocida toxin (PMT). The aim of this study was to investigate the efficacy under field conditions of a vaccine intended for the prevention of NPAR (Rhiniseng®) on a Spanish farm with Bb-associated problems.
Materials and Methods
A farrow-to-nursery pig herd was selected for a controlled clinical trial, on the basis of clinical signs and laboratory tests for Bb in the nursery. Four pregnant sows were primo-vaccinated with Rhiniseng® (Group V), while other four received PBS (Group NV). The parameters evaluated in their offspring were, presence of serum antibodies against Bb and PMT, presence of Bb antigen in nasal discharge, and clinical signs of respiratory disease. In addition, the NPAR-related macroscopic and microscopic lesions [4,5], and weight gain were also assessed at 6.5 and 9.5 weeks of age, respectively.
Results and Discussion
Immunisation of pregnant sows can prevent the development of atrophic rhinitis in their offspring [6]. This study showed the suitability of a bivalent vaccine (Bb + PMT) for controlling NPAR in piglets, after vaccination of their Bb-seropositive/PMT-seronegative dam in a herd with Bb-related disease.
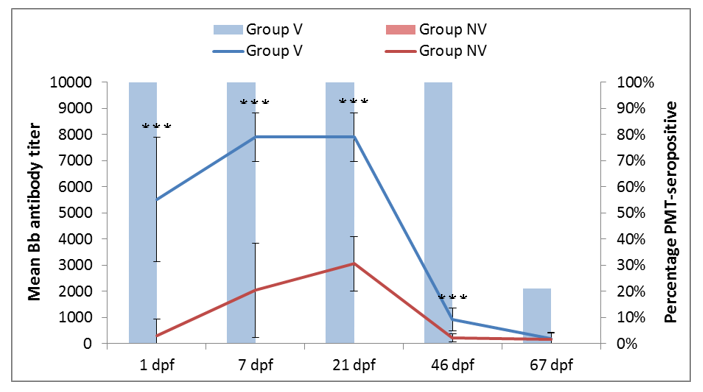
Figure 1. Mean Bb antibody titer in the vaccinated (blue line) and non-vaccinated (red line) piglets and percentage of PMT seropositive piglets in the vaccinated (blue bars) and non-vaccinated (red bars) groups as a function of the days post-farrowing (dpf). Statistically significant differences (P values<0.001) at the same time point are indicated (***).
Colostrum intake ensured an efficient transfer of antibodies against Bb and PMT to piglets (Figure 1). Significant differences of antibody titers were observed between piglets in Group V and Group NV for all vaccine antigens. It is very likely that this fact has resulted in the decrease of Bb prevalence in piglets in Group V compared to Group NV (Figure 2), hence, the vaccination could decrease the Bb burden and help to prevent clinical outbreaks [3].
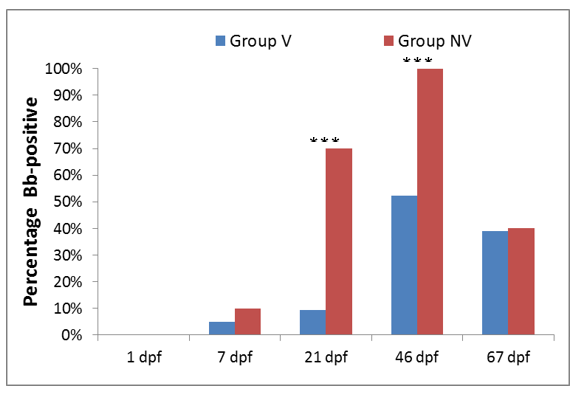
Figure 2. Percentage of Bb shedders in the vaccinated (blue bars) and non-vaccinated (red bars) groups. Statistically significant differences (P values <0.001) at the same time point are indicated (***).
No clinical respiratory signs were observed in any of the piglets studied. However, a higher percentage of piglets in Group NV had macroscopic lesions compatible with NPAR, higher mean nasal lesion scores, and more evident bone alterations compared with those in Group V (Table 1). Finally, the mean body weight per piglet was also higher in Group V than in group NV (20.46 kg vs. 18.56 kg, respectively).
Table 1. Macroscopic and histological lesions in turbinate and nasal septum at 6.5 weeks of age in the groups studied.
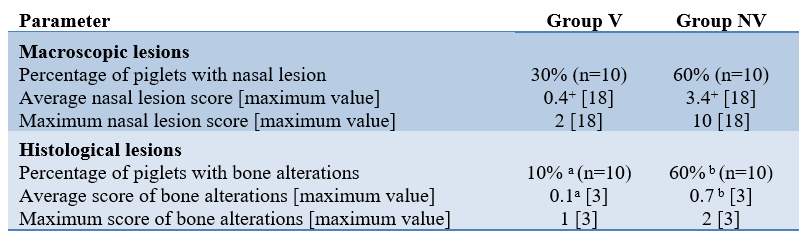
*a,b: Statistically significant differences (P values <0.05) at the same parameter are indicated with different superscripts; + Statistical differences level (P values = 0.09).
Conclusions
Immunisation of pregnant sows with Rhiniseng® prior to farrowing induced a high concentration of serum antibodies, which were efficiently transferred to piglets by colostrum. This maternal immunity most likely contributed to the reduction of the Bb infection burden, and consequently to the decrease of atrophic rhinitis lesions. It is also likely that the implementation of a vaccination program in this farm had a beneficial effect on productive parameters during weaning and nursery periods.
By A. Sánchez-Matamoros,1 PhD; A. Martos,1 MSc; O. Boix,1 MSc; A. Martínez-Abarca,2 DVM; F.J. Pallarés,3 PhD 1 HIPRA, Girona, Spain; 2 Dalland Hybrid España S.A., Murcia, Spain; 3 Dept. Anatomy and Comparative Pathology, Faculty of Veterinary Medicine, Murcia University, Murcia, Spain
Acknowledgements
The authors would like to acknowledge Ramón Riquelme and Ramon Jordà for their technical assistance and UCAM staff for trial monitoring and laboratory support.
References
1 Official Journal of the European Union, 2015. C 299/04.
2 Sneeringer S et al. 2015. ERR-200.
3 Brockmeier SL et al. 2012. Diseases of swine; 49:670-679.
4 European Pharmacopoeia 8.0. Monograph 04/2013:1361.
5 Brockmeier SI et al. 2002. Infect. Immun;70(2):481–490
6 Foged NT et al. 1989. Vet. Rec;125(1):7–11.







