Imuvant - A Novel Adjuvant; Efficacy and Safety Properties in Hyogen Vaccine
The aim of this study by Ceva was to assess the safety of Hyogen® and evaluate the boosting effect of the J5 component of Imuvant™ on the efficacy of Hyogen® vaccine in 3-week-old piglets, write Miklós Tenk, Anna Kollár, Ferenc Misák, Mariann Ivók, Nimród Pálmai and Zoltán Pénzes, Ceva.In previous studies the safety and potency of several adjuvants, such as carbomer 974, DDA, squalene, Montanide ISA 206, competitors’ swine vaccine adjuvants and Imuvant™, a proprietary Ceva adjuvant were evaluated in pigs by using a model antigen.
Based on these earlier results, only Imuvant™ was both safe and immune-potentiating.
Subsequently, Imuvant™ was selected to be the adjuvant for Hyogen®, a bacterin vaccine for the prevention of enzootic pneumonia caused by Mycoplasma hyopneumoniae (M.hyo). Imuvant™ consists of mineral oil and Escherichia coli J5 non-toxic LPS, which significantly boosts both the cellular and the humoral immune responses.
The aim of the current study was to assess the safety of Hyogen® and evaluate the boosting effect of the J5 component of Imuvant™ on the efficacy of Hyogen® vaccine in 3-week-old piglets.
Materials and Methods
Safety Three different batches of Hyogen® vaccine were injected into 3-week-old seronegative piglets under placebo control.
In addition to monitoring the systemic and local reactions to the vaccines, measuring body temperature increase at several time-points, aspartate amino¬transferase (AST) and creatine kinase (CK) blood biochemical tests were also performed as being fine indicators of possible tissue damage due to vaccination.
Efficacy Forty-five (45) piglets seronegative to M.hyo were divided into 3 groups of 15 and then vaccinated with the following vaccines: (i) Hyogen®: M.hyo bacterin adjuvanted with Imuvant™ (containing J5), (ii) M.hyo bacterin adjuvanted with mineral oil only, no J5 added and (iii) Placebo.
Eighteen (18) days after vaccination, the pigs were challenged with a virulent M.hyo strain. At 28 days post challenge the animals were slaughtered and subjected to lung scoring according to the European Pharmacopoeia.
Tracheal swab samples were also taken for real time PCR analysis to determine the level of colonization of the lungs by the M.hyo challenge strain.
Results
Injection of three batches of Hyogen® into 3-week-old piglets revealed no major systemic or local reaction to the vaccines. The rectal temperatures remained in the normal range. The AST and CK enzyme analysis indicated no tissue damage due to the vaccination.
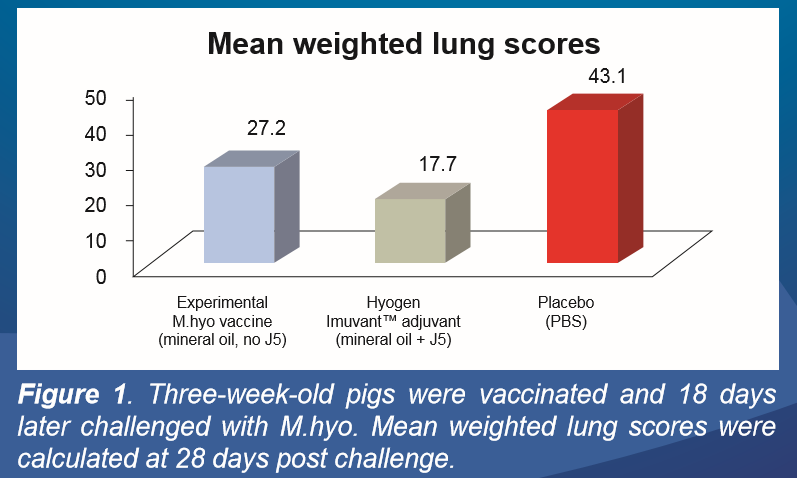
Based on the evaluation of lung lesion at 28 days post M.hyo challenge, a clear advantage of Imuvant™ (mineral oil + J5) over the mineral oil adjuvanted vaccine was observed (Figure 1).
Hyogen®, adjuvanted with Imuvant™ significantly reduced the lung lesions compared to the placebo group (p=0.028). The mineral oil adjuvanted M.hyo vaccine (containing no J5) also reduced the lung lesion scores but statistical significance could not be demonstrated against the placebo group (p=0.288).
The real time PCR analysis of the trachea swab samples confirmed the benefit of J5 in Hyogen®, indicating that the level of M.hyo challenge strain colonization was lower with J5, compared to mineral oil adjuvant only.
Conclusions
In summary, this study demonstrated the excellent safety of Imuvant™, and confirmed that J5 non-toxic LPS is a key component to enhance the efficacy of Hyogen®, compared to an experimental M.hyo bacterin adjuvanted with mineral oil only.
Hyogen®, adjuvanted with Imuvant™ (containing mineral oil + J5), provided protection against M.hyo challenge as early as at 18 days post vaccination.