



Comparative study of two vaccines against neonatal diarrhoea on a Canadian commercial farm
Vaccine exhibits 6-fold lower incidence of diarrhoea in suckling piglets from gilts vaccinated with it than in suckling piglets from gilts vaccinated with its competitor..jpg)
Introduction
Infection with pathogenic Escherichia coli is a common cause of diarrhoea in suckling pigs worldwide and is becoming an important and devastating disease for swine producers. It is also responsible for a substantial economic impact on farms worldwide, due to high morbidity, mortality and reduced growth rates. [1] In some studies, conducted in Europe, diarrhoea is estimated to account for 5-24% of overall pre-weaning mortality and to reduce average daily gain (ADG) by 8-14 g per day. [2,3] Based on these effects, the cost of neonatal diarrhoea was recently estimated to be €134 per sow per year. [4] There are different products on the market that will help us to control neonatal diarrhoea through vaccination. Suiseng®, which is one of the vaccines involved in this trial, is a subunits vaccine that contains purified adhesion factors (F4ab, F4ac, F5 and F6) and the heat-labile toxin (LT) of Escherichia coli, the β toxin of Clostridium perfringens type C, to prevent neonatal diarrhoea in piglets through passive immunisation, and the α toxin of Clostridium novyi, to protect sows suffering from sudden death by active immunisation. Moreover Suiseng® includes a new, state-of-the-art adjuvant, Hipramune® G, based on ginsenosides, saponins from ginseng root, which enhances an animals’ antigen presentation process. [5] An increase in the number of APCs (antigen-presenting cells) and the amount of antigen on their surface is one of the keys in the ability of adjuvants to increase the effectiveness of vaccines. [6] On the other hand, the other vaccine used in this comparative study, Vaccine B, which is a pure bacterin, contains Escherichia coli adhesion factors F4, F5, F6 and F41, and the β toxin of Clostridium perfringens type C, with an aluminium hydroxide adjuvant.
The aim of this study was to assess and compare the safety and efficacy performance of two commercial vaccines registered in Canada, under field conditions, on a commercial farm where E. coli was found to be the source of diarrhoea.
Materials and methods
Experimental design and vaccination regimens
Sixty gilts were selected for this study from a farrow-to-wean farm with 1000 sows, located in Manitoba (Canada). Since the farm had a 4-week batch farrowing system, two consecutive batches of 30 gilts were selected and randomly divided into two groups. The first batch was divided into groups 1A, n=15, Vaccine A (Suiseng®) and 1B, n=14, Vaccine B, and the second batch was divided into groups 2A, n=15, Vaccine A (Suiseng®) and 2B, n=15, Vaccine B. The gilts enrolled in each group were identified by a spray marker, as were the piglets that those sows adopted from other sows not included in the study; they were ear notched so as not to include them in the final production parameters.
Among the selection criteria of the farm included in the protocol, the commercial farm under study had to be one affected by neonatal diarrhoea caused by colibacillosis, i.e., more than 5% of the litters had to be affected by E. coli or Clostridium perfringens induced neonatal diarrhoea.
Fecal samples from piglets suffering from diarrhoea were sent from the candidate farm to Hipra’s Diagnos® Laboratory (Spain). Thus, samples from 9 piglets were sent on 3 FTA® (Flinders Technology Associates; filter paper-based DNA assay) ELUTE cards (Whatman Inc., Florham Park, NJ). A multiplex polymerase chain reaction (PCR) test, adapted from previous studies, [7,8] was performed to detect genes encoding F4, F5, F6 adhesion factors and LT of E. coli, and the β-toxin of Clostridium perfringens type C.
The vaccination protocol consisted of two doses by the intramuscular route 6 and 3 weeks before farrowing. Primary vaccination and revaccination were administered to the first batch on April 4th and 25th 2016, respectively; and May 2nd and 23rd 2016 in the second batch.
All the procedures described were performed following the guidelines from the Canadian National Farm Animal Care Council – Code of practice for the care and handling of pigs.
Safety and efficacy
Several parameters were assessed to evaluate and compare the safety and efficacy of both products. The first safety parameter evaluated was the body temperatures of all the animals involved in the study in relation to the first vaccination and revaccination. Temperatures were taken at the following times: the day before vaccination (-24h), time before vaccination (0h), 6 hours after vaccination (6h), one day after vaccination (24h) and two days after vaccination (48h). The basal temperature was obtained from the mean of temperatures recorded on -24h and 0h. These data were used to obtain the mean body temperatures of each of the groups and the temperature increases produced by vaccination.
The second parameter tested was that of local reactions at the injection site and two variables were used: Swelling or No swelling.
Lastly, the third parameter evaluated was that of general type reactions, using 7 different variables: Type 1 prostration, Type 2 prostration, Type 3 prostration, Anorexia, Death, Abortion or No systemic reaction.
To evaluate efficacy, piglets from gilts vaccinated with both products were monitored throughout the lactation period (mean of 19.49 ± 1.35 days). The following were evaluated as primary parameters: litters affected by diarrhoea and piglets affected by diarrhoea. The secondary parameters evaluated were: mortality of suckling piglets, the daily average weight gains of those piglets and, if they had diarrhoea, to determine its causes.
Statistical methods
A statistical analysis of the results was undertaken at the University of Girona Statistics Department to compare the results of both the safety and efficacy of the vaccine. The statistical analysis consisted of a case-control study. SPSS software v.19 (SPSS Inc., Chicago, IL) was used to read, process and obtain results.
In the event that the quantitative variables were normal, a Student’s t-test was used to compare the null and alternative hypotheses. In the case of non-normality, a non-parametric Mann-Whitney U test was used to compare the medians. The contingency table for two qualitative variables and chi-square test were used for qualitative variables to compare whether the two variables were independent or were related. Data are expressed as mean ± S.E.M. Statistics decisions were made using a significance level value of 0.05.
Results
Temperature measurements
Regarding the comparison of safety, the mean body temperature after vaccination always remained lower in the groups vaccinated with Suiseng® than in the groups vaccinated with Vaccine B. The mean body temperatures of all the groups before and after vaccination are summarised in Figure 1.
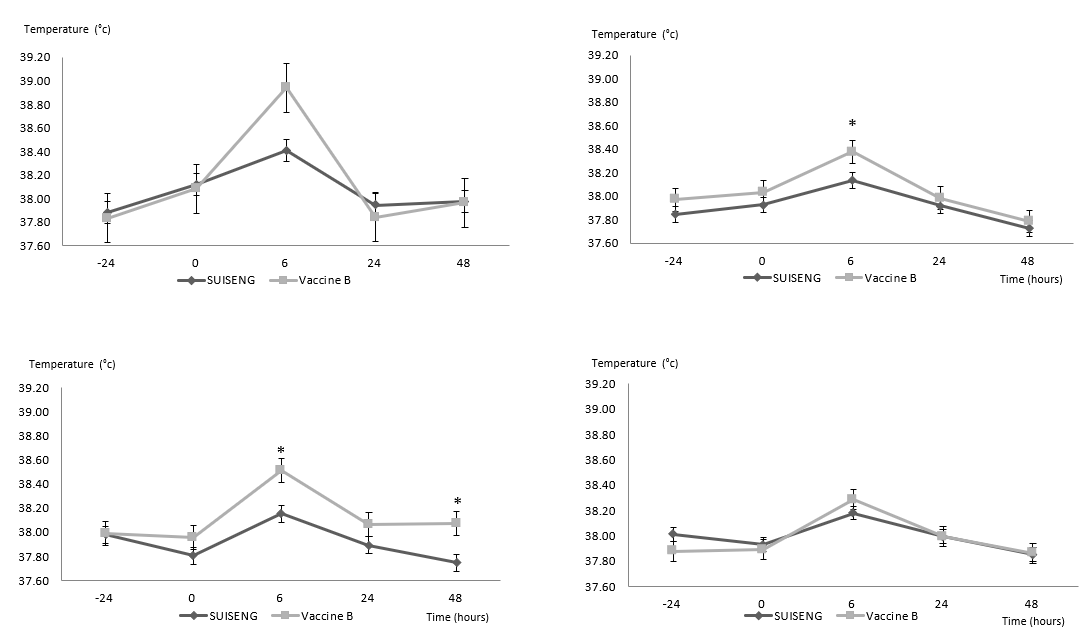
Figure 1: The effect of vaccination on the body temperature of gilts vaccinated with Vaccine A (Suiseng®) or Vaccine B. Temperatures (ºC) was monitored the day before vaccination (-24h), time before vaccination (0h), 6 hours after vaccination (6h), one day after vaccination (24h) and two days after vaccination (48h). Body temperatures of (A) batch 1 gilts after first vaccination, (B) batch 1 gilts after revaccination, (C) batch 2 gilts after first vaccination, and (D) batch 2 gilts after revaccination are shown. Values represent the mean ± SEM of 14-15 animals per group. Statistically significant difference *P < 0.05, ***P < 0.001.
In the first batch vaccinated (baseline temperature was 37.98 ºC at the first vaccination and 37.95 ºC at revaccination), statistically significant differences were found at 6 hours after the first vaccination (Figure 1A): 38.41 ºC in 1A and 38.94 ºC in 1B (Mann-Whitney U test, P-value < 0.001) and 6 hours after revaccination (Figure 1B): 38.13 ºC in 1A and 38.40 ºC in 1B (Student's t-test, P-value < 0.05).
In the second batch vaccinated (baseline temperature was 37.94 ºC at the first vaccination and 37.93 ºC at the time of revaccination), statistically significant differences were found 6 hours after the first vaccination: 38.15 ºC in 2A and 38.51 ºC in 2B (Student's t-test P-value < 0.05) and 48 hours after the first vaccination (Figure 1C): 37.75 ºC in 2A and 38.10 ºC in 2B (Student's t-test, P-value < 0.05).
Regarding the body temperature increases that occurred after vaccination (data not shown), group 1B showed a mean increase of 0.98 ºC 6 hours after the first vaccination, whereas group 1A showed a mean increase of 0.41 ºC (Student's t-test, P-value < 0.05).
In relation to the other monitored parameters relating to safety, no local type reactions were reported in either group. With regard to general type reactions, results for all the groups are summarised in Table 1.
Table 1: Summary of gilts’ general adverse reactions recorded after first vaccination and revaccination with Vaccine A (Suiseng®) or Vaccine B.
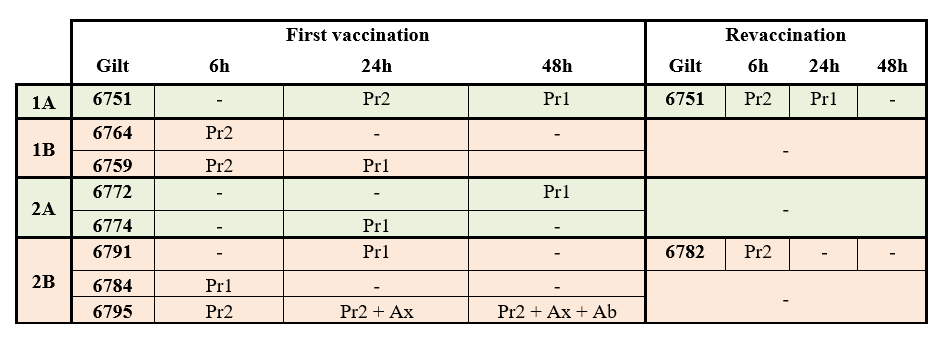
-, No systemic reactions; Ab, Abortion; Ax, Anorexia; D, Death; Pr, Prostration; Pr1, Inactive but responds to weak stimuli; Pr2, Does not respond to weak stimuli but does to strong ones; Pr3, Does not respond to strong stimuli.
Remarkable general type reactions were only observed in one animal in group 2B that began with grade 2 prostration (response only to strong stimuli) and anorexia by 6 and 24 hours, respectively, after the primary vaccination. Subsequently, the animal exhibited vaginal discharge by 48 hours’ post-vaccination and finally aborted its fetuses (16 fetuses were counted) (Figure 2).
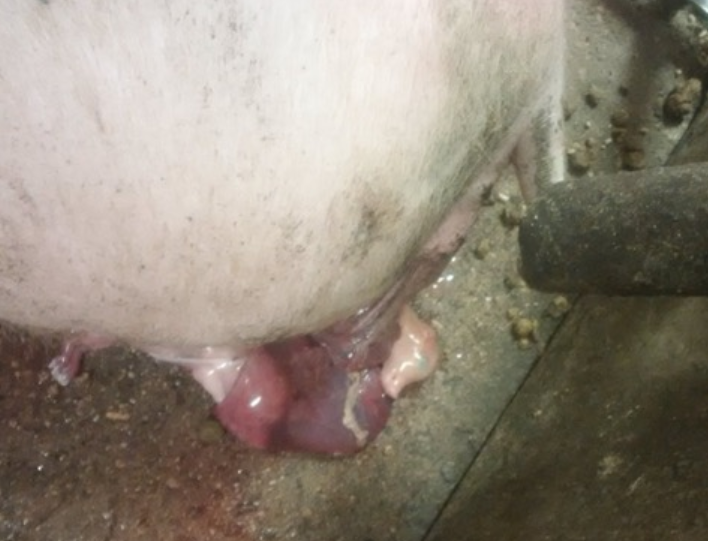
Figure 2: Vaginal discharge image from one animal 48 hours post primary vaccination with Vaccine B. The gilt finally aborted 16 fetuses.
Incidence of neonatal diarrhoea
The 3 FTA cards evaluated were positive for the E. coli F4 adhesion factor; thus, in the end, it was decided to include this candidate farm in the study.
The results from litters and piglets with diarrhoea were evaluated to compare vaccination efficacy. In the first batch, two litters from group 1A were affected (13.4% of litters) amounting to a total of 3 affected piglets (1.6% of the total of weaned piglets in the group). In group 1B, five litters were affected (35.7% of litters) amounting to a total of 39 animals (22.4% of the total number of piglets in the group).
In the second batch of gilts, a total of 7 piglets were affected by diarrhoea (3.8% of all weaned piglets in the group) in two litters (13.4% of litters) in group 2A. In group 2B, 25 animals were affected by diarrhoea (18.2% of weaned piglets in the group) in six litters (46.1% of litters).
The piglets with diarrhoea did not require any specific antibiotic treatment and they were simply administered potato starch as an alternative approach to limit symptomatic incidence of scouring. In this study, the use of antibiotic was not at all needed, with no detrimental concerns on animal welfare. In addition, faecal samples were taken from 21 piglets that had diarrhoea in both vaccinated groups and analysis showed that 86% of the samples were positive for F4, 57% were positive for F5 and 29% of the samples were positive for LT.
Production parameters
Production parameters are presented in Table 2. There were no substantial differences between groups regarding mortality and most animal deaths were due to them being non-viable or crushed. The reduced trend in born alive piglets, birthweight, weaned piglets and weaning weight in group B was due to the fact that there was initially one animal fewer in group 1B (random group split up) and two fewer animals in group 2B (one aborted 48 hours after the first vaccination and one animal euthanized at farrowing date due to dystocia).
Table 2: Summary of productive parameters obtained in sows vaccinated with Vaccine A or Vaccine B.
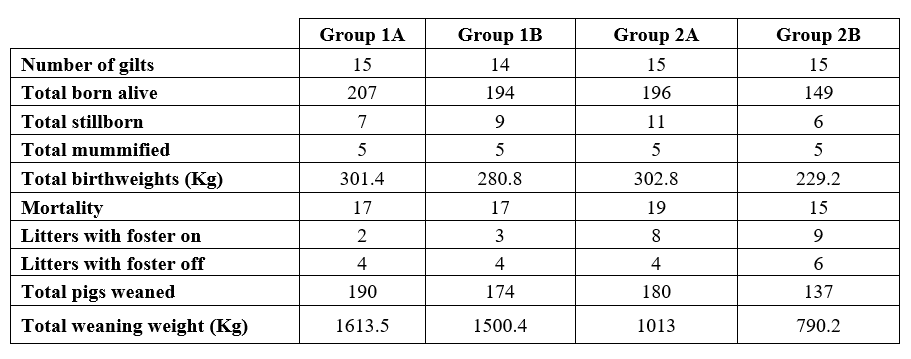
1A, batch 1 animals vaccinated with Vaccine A (Suiseng®); 1B, batch 1 animals vaccinated with Vaccine B; 2A, batch 2 animals vaccinated with Vaccine A (Suiseng®); 2B, batch 2 animals vaccinated with Vaccine B.
Conclusions
Neonatal diarrhoea currently continues to be one of the pathologies that has the highest cost for producers.4 E. coli is still one of the primary triggers among the infectious agents that cause neonatal diarrhoea in pigs. [9] Thus, current production conditions and the restrictions on the use of antibiotics to which we are subject, mean that, nowadays, vaccination of pregnant sows for controlling neonatal diarrhoea is widespread. [9] This is largely due to the excellent results obtained when it is combined with good hygiene and management practices. [10]
The use of state-of-the-art vaccines that incorporate innovative adjuvants and new purification techniques for antigens are essential in cases where there is a high infection pressure or chronic colibacillosis. [10] It has previously been demonstrated that vaccination is an effective strategy to reduce mortality due to neonatal diarrhoea on high infection pressure farms. [11] Along the same lines, the current study shows differing diarrhoea-preventive effects of the two vaccines evaluated. Thus, Vaccine A (Suiseng®) exhibited a 6-fold lower incidence of diarrhoea in suckling piglets from gilts vaccinated with it than in suckling piglets from gilts vaccinated with its competitor, Vaccine B. Moreover, Vaccine A (Suiseng®) showed greater safety than Vaccine B by causing a smaller increase in post-vaccination body temperature and by not showing any local or general type reactions, which may end in abortion and the resultant economic loss to the producer.
The current comparative evaluation indicates that immunisation of sows with Suiseng® can effectively protect their offspring against neonatal E. coli diarrhoea.
By W. Ariza,1 DVM; D. Robles,1 BSA; J. Badia,2 DVM; D. Torrents,3 PhD; I. Bernal,3 DVM. 1 Swine Health Professionals, Steinbach, Manitoba (Canada); 2 Hipra Animal Health Canada Inc., Ottawa, Ontario (Canada); 3 Laboratorios Hipra S.A, Amer, Girona (Spain).
Acknowledgment
The authors would like to thank the Swine Health Professionals technical team for their help and cooperation in monitoring and carrying out tasks, the University of Girona Statistics Department for the statistical analysis performed, and the Diagnos® laboratory for the analytical support given in conducting this study.
References:
1. Shi, D. et al. Frequency of virulence factors in Escherichia coli isolated from suckling pigs with diarrhoea in China. The veterinary journal. 2014;199:286–289.
2. Westin, R. et al. Post-mortem findings and piglet mortality in relation to strategic use of straw at farrowing. Preventive Veterinary Medicine. 2015;119(3-4):141-152.
3. Kongsted, H. et al. The effect of New Neonatal Porcine Diarrhoea Syndrome (NNPDS) on average daily gain and mortality in 4 Danish pig herds. BMC Veterinary Research. 2014;10(1):90.
4. Sjölund, M. et al. Financial impact of disease on pig production. Part III. Gastrointestinal disorders. Proceedings of 6th ESPHM, Sorrento, Italy. 2014;189.
5. Rivera E. Ginseng extract in aluminium hydroxide adjuvanted vaccines improves the antibody response of pigs to porcine parvovirus and Erysipelothrix rhusiopathiae. Veterinary immunology and immunopathology. 2003;91(203):19-27.
6. Chase C. Swine immunology: What does it take to make the immune response take? AASV. 2012;411-416.
7. Zhang W, et al. Prevalence of virulence genes in Escherichia coli strains recently isolated from young pigs with diarrhoea in the US. Vet. Microbiol. 2007;123(1-3):145-52.
8. Baums C, et al. Diagnostic multiplex PCR for toxin genotyping of Clostridium perfringens isolates. Vet. Microbiol. 2004;100(1-2):11-6.
9. Melkebeek V. et al. ETEC vaccination in pigs. Veterinary Immunology and Immunopathology. 2013; 152:37-42.
10. Guerra N. et al. Efficacy of Suiseng®: a novel friendly adjuvanted vaccine against neonatal colibacilosis and clostridiosis. Proceedings of the 21st IPVS Congress, Vancouver, Canada, 2010;778.
11. Bernal I. et al. Field study to assess the economic benefit of vaccination with Suiseng® on a Portuguese farm. Proceedings of the 24th IPVS Congress, 8th ESPHM, Dublin, Ireland, 2016;205.







