



Emergence of Porcine Epidemic Diarrhoea in North America
A good review of current knowledge of porcine epidemic diarrhoea by C. Hill, E. Raizman, T. Snider, S. Goyal, M. Torremorella and A.M. Perez for the FAO.Abstract
Porcine epidemic diarrhoea virus (PEDv) was first recognised in the United Kingdom of Great Britain and Northern Ireland in 1971. Since then, PEDv has become endemic in many European and Asian countries, where PEDv infection typically results in minor outbreak incidents with relatively low mortality (Pan et al., 2012; Li et al., 2012b).
In 2010, despite vaccination programmes, a virulent strain of PEDv caused high mortality in neonatal piglets in Chinese herds and economic losses continue to mount (Geiger and Connor, 2013).
The first outbreak of virulent PEDv in North American swine herds occurred in the US in May 2013 (Ackerman, 2013). During the first months of the epidemic, PEDv losses contributed to a decline in the national market of between 2.5 and 4.2 per cent (Meyer, 2013).
The full economic impacts of PEDv losses in United States and Chinese pork markets have not been measured, and current efforts to prevent further losses focus on prevention and control strategies.
Background Information
Global trade of live pigs, pig embryos, semen, feed ingredients and feed is relatively common, leading to a threat of transboundary PEDv transmission into the swine populations of naïve countries. It is worth noting that PEDv does not pose a public health risk as it does not cause disease in humans. However, PEDv can affect the livelihoods of producers in affected regions because of the severe and far-reaching economic losses that the disease imposes. These losses are particularly critical for small-scale farms where a high mortality rate can be devastating to food security, especially in developing countries.
Coronaviruses are found worldwide and PEDv has been reported in 11 of the top 20 swine-producing countries. PEDv is prevalent in some European swine herds although current prevalence levels are unknown and it is believed that the disease does not cause major economic losses in Europe (Pan et al., 2012; Song and Park, 2012).
PEDv has been a cause of enteric diarrhoea in Chinese piglets since 1973; the use of a killed vaccine appears to have reduced outbreak levels (Li et al., 2012b; Chen et al., 2010).
Sporadic outbreaks occurred in Japan, the Republic of Korea, Thailand and Viet Nam during the 1990s (Pan et al., 2012) but prevalence did not appear to be widespread.
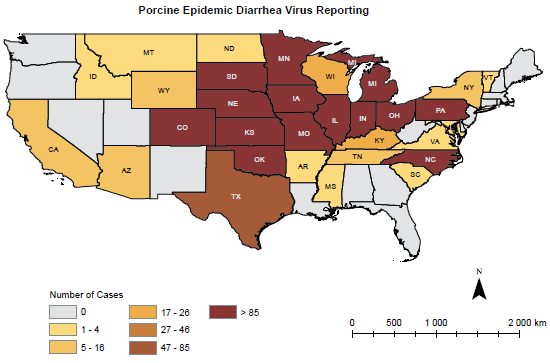
NOTE: 0 No positive accessions reported; 1-4 Negligible number of accessions reported; 5-16 Minor number of accessions reported; 17-26 Mild number of accessions reported; 27-46 Moderate number of accessions reported; 47-85 Marked number of accessions reported; 86 Severe number of accessions reported.
Source: Swine Health Monitoring Project, University of Minnesota.
This situation of apparent equilibrium changed in 2010, when bouts of watery diarrhoea occurred in Chinese herds that had been vaccinated against PEDv (Pan et al., 2012). It appeared that a new variant of PEDv was causing high mortality in suckling piglets in several regions of China (Pan et al., 2012; Sun et al., 2012). Thailand also experienced outbreaks of virulent PEDv during this time (Puranaveja et al., 2009), with close to 100 per cent mortality in neonatal piglets (Song and Park, 2012). In May 2013, outbreaks of watery diarrhoea and high piglet mortality were noted in several North American herds, and PEDv has increased in prevalence since then (Huang et al., 2013; Geiger and Connor, 2013).
To date, the prevalence of PEDv ranges from 30 to 40 per cent in European swine herds (AASV, 2013; Van Reeth and Pensaert, 1994), with higher prevalence occurring on smaller sow farms, and is 43 per cent on Chinese hog farms, where prevalence is higher in breeding herds (Carvajal et al., 1995; Li et al., 2012a).
Most field strains found in Asia are genetically related (Pan et al., 2012; Song and Park, 2012; Chen et al., 2010; Bi et al., 2012), but genetically different from the Chinese vaccine strain and from the strain endemic in Europe (Chen et al., 2010). Strains isolated from the states of Minnesota and Iowa in the United States of America are almost identical and share unique nucleotide sequences with virulent Chinese strains (Huang et al., 2013). Since then, much has been learned about PED in North America.
This report represents the state of knowledge at the time when it was written (July 2014). However, readers should be aware that knowledge of PED immunity, pathogenesis and epidemiology is evolving rapidly in North America and it is possible that some of the features presented here may be out of date.
Quick Facts
What is PEDv?
PEDv is an enveloped, ribonucleic acid (RNA) coronavirus (Pan et al., 2012; Song and Park, 2012; Huang et al., 2013). In suckling pigs, PEDv causes similar disease to transmissible gastroenteritis virus (TGEv), but the two viruses belong to separate subgroups of the coronavirus family (Pan et al., 2012).
How do pigs get PEDv?
The faecal–oral route but many different modes of transmission are possible through either direct or indirect contact, including through the sow’s milk, aerosol droplets and feed (Sun et al., 2012; Geiger and Connor, 2013).
Large amounts of virus are shed in the faeces as early as two days post-infection and for up to 28 days post-infection (Hesse, 2013). PEDv survives in fresh faeces for at least seven days at varying temperatures and humidity levels.
PEDv can also be shed in nasal secretions but the amount depends on the viral load and the presence of viraemia (Hesse, 2013). Fomites also promote spread of the virus, as the virus is present in the environment and on equipment and clothing.
Which pigs die from PEDv?
Suckling piglets under two weeks of age are most at risk of mortality from PEDv where they are in close contact with litter mates and sows and rely completely on milk for nutrition (Pan et al., 2012; Li et al., 2012b; Sun et al., 2012). Virulent strains appear to have pathological effects on small intestinal villi, with blunted and fused villi developing in the jejunum and ileum (Kim and Chae, 2000).
Piglets present with watery diarrhoea, dehydration and vomiting approximately five to six days post-infection (Pan et al., 2012; Li et al., 2012b; Sun et al., 2012). Severe enteric disease eventually results in weight loss, decreased food consumption and lethal dehydration and emaciation (Martelli et al., 2008). The survival rate of piglets increases if they live to seven days post-infection (Ackerman, 2013).
How is PEDv infection diagnosed in pigs?
A rapid, sensitive and cost-effective method of screening for PEDv during an outbreak of acute gastroenteritis is testing the faeces of infected pigs by reverse transcription polymerase chain reaction (RT-PCR) (Song and Park, 2012). Ideal samples for submission are from live piglets in acute stages of disease (Geiger and Connor, 2013). Fresh faeces from pigs with active, watery diarrhoea should also be submitted for PCR (AASV, 2013).
A PCR multiplex assay for PEDv and TGEv has been developed and is available through many laboratories worldwide (Chen et al., 2010). PCR kits for PEDv can be obtained from corporations but may not be readily available on a global scale.
An enzyme-linked immunosorbent assay (ELISA) has been developed to test herds for exposure to PEDv by detecting anti-PEDv antibodies but this is not a farm-based test and is not available in all countries. In addition, although ELISA can test exposure two to four weeks post-infection, it cannot test acute infection or exposure more than a month post-infection.
Immunofluorescent antibody testing can be performed in some countries but the laboratory must have the capacity to grow the virus in vitro and have trained staff to interpret the results under a fluorescent microscope.
To date, PEDv RT-PCR is the most sensitive and rapid way of diagnosing PEDv infection in a herd.
Disease Impact
To date, the virulent strains of PEDv that caused high mortality in Asia, the US and Canada are not highly prevalent elsewhere in the world.
Although Europe has experienced outbreaks of PEDv, the strain has not been very virulent and the outbreaks have resulted in low mortality in piglets. Other large pork-producing countries, such as Brazil and the Russian Federation, have not reported cases of PEDv in their commercial herds. However, according to Web-based reporting systems, PEDv has been diagnosed in smaller pork-producing countries, such as Colombia, the Dominican Republic, Mexico and Peru.
The full economic impact of PEDv outbreaks in North America since May 2013 cannot yet be calculated. The United States pork market is worth more than US$97 billion, and losses are estimated at five million piglets over recent months (WATTAgNet, 2014). The total number of hogs going to market in 2014 will be three per cent lower than in 2013, with prices increasing by three per cent from 2013 to 2014 (Campbell and Chen, 2014).
Chinese hog markets do not appear to be growing as rapidly as expected, even though more farms are applying intense management systems and improving biosecurity. However, most authorities believe that China’s pig numbers are still increasing because diseases that result in high mortality (such as PEDv) or culling (such as foot-and-mouth disease virus) are skewing the total inventory of pigs in China (ThePigSite, 2013). The profits of the hog industry in China have also not rebounded as expected, and the price per pig has increased, putting a strain on the average Chinese consumer (ThePigSite, 2013).
Epidemiological Outlook
In addition to faecal–oral and direct nose–nose transmission, new research has shown that PEDv can be infective in feed and in airborne particles; piglets have developed diarrhoea from these sources and their intestinal tracts tested positive for PEDv seven days post-infection (Alonso et al., 2014).
As mentioned in the section on Quick facts, fomites can serve as routes for the spread of PEDv between farms, when veterinarians, visitors and haulers transporting hogs to market carry PEDv-infected faeces from one farm to another.
The type of production system and the pig density contribute to increased risk of PEDv infections, with farrow-to-wean sites having greater risk of PEDv infection than wean-to-finish sites, and high pig density increasing PEDv risk compared with lower pig density (USDA, 2014b).
Additional risk factors include increased number of trash pick-ups, increased visits from rendering trucks, more pigs entering herds from outside sources, introduction of equipment borrowed from another farm, and presence of wildlife on farms (USDA, 2014a; 2014b).

A majority of high-producing pork states in the US, several provinces in Canada and most of the high producing pork countries in Asia have recently documented cases of the virulent form of PEDv.
Most high producing pork countries have had outbreaks of TGEv, and it seems likely that a similar pattern of PEDv outbreaks could occur in these countries if import restrictions and biosecurity are not improved.
Both diseases are coronaviruses, and both cause high mortality in neonatal piglets. In many countries, it used to be believed that TGEv was a foreign disease but this may not be true today.
The situation may be similar for PEDv. Following the interest caused by the PEDv epidemic in the US, several Latin American countries, such as Colombia, the Dominican Republic, Mexico and Peru, have reported the disease, suggesting that PEDv may be more widespread than previously thought.
Raising awareness of the disease will therefore help to improve surveillance, early detection and reporting of the disease in other countries, where small-scale farms in the swine sector are the most affected because of the virus’s devastating effects on farmers’ livelihoods.
Response and Action
Although PEDv is not zoonotic and is not present in meat, many countries have imposed a ban on pork products originating from PEDv-positive herds and regions because of the risk of contamination and indirect transmission. Mexico imposed a temporary ban on US pig imports in July 2013 (Daily Livestock Report, 2013), and the Russian Federation has temporarily restricted the import of pigs from several countries that are reporting PEDv (Food Safety News, 2014).
These bans result from concerns over introduction of the virus into these countries’ domestic herds.
A risk assessment conducted by the Province of Quebec in Canada concluded that the risk of PEDv introduction into Canada from the US was high for returning trucks, and moderate for other routes such as feed and feed sub-products. To prevent outbreaks, biosecurity protocols have been increased for most farms in the United States of America and Canada, and the identification of risk factors for individual farms assists the farms in adjusting biosecurity measures that reduce the risk of introduction of PEDv into their herds.
Recommendations for Prevention and Control
Early diagnosis of PEDv is the best way of decreasing piglet mortality. If PEDv is diagnosed at the first onset of acute enteric disease, exposure and elimination protocols can be enforced immediately to increase piglet survival in the future (Geiger and Connor, 2013; AASV, 2013).
During the initial outbreaks in the US, producers on farms or in areas with disease were urged to expose pre-farrowing sows to PEDv to ensure passive immunity for their piglets during the suckling period. In an attempt to eliminate disease completely, entire herds were closed and exposed to the virus. Replacement gilts and sentinel animals were used to propagate the herd and test for elimination of the virus. This procedure, called 'feedback', is described in the following section (Geiger and Connor, 2013).
Currently, only one vaccine has been approved for commercial use in the US (Harris Vaccines, 2014; USDA, 2014c). The Chinese commercial vaccine is not protective for the virulent strain but a vaccine for the virulent strain is being developed in China. The methods most frequently used for reducing, controlling and preventing the disease are exposure, sanitation and biosecurity (Geiger and Connor, 2013).
Improved biosecurity can also prevent outbreaks of PEDv on farms after the risk factors for PEDv introduction have been assessed. Improved biosecurity includes additional biosecurity checkpoints for visitors and personnel entering farms, and provision of off-site drop-off or pick-up points for trucks and of special entries on to the farm for the drivers of these trucks (USDA, 2014b).
Reducing the presence of rodents, birds and raccoons on farms will reduce risk of the spread of PEDv on to and within farms. Although most US commercial herds are kept in closed barns, cracks in ventilation areas and broken windows or doors can contribute to the presence of rodents and birds.
In other pork-producing countries, where only semi-intensive systems are in place, rodent and wildlife have easy access to the pigs. Restricting the access of wildlife to the pigs can greatly enhance biosecurity and prevent spread of disease, including PEDv (USDA, 2014b).
Disease Elimination: Feedback
Feedback (Geiger and Connor, 2013; Schwartz et al., 2013) is a method of exposure to and elimination of diseases that cause high mortality in piglets.
Although animal rights activists have expressed concerns about this method, the motivation for its use is to mitigate the consequences of a disease. In fact, in many aspects, feedback resembles the strategy followed in the early history of vaccine development, when healthy individuals were intentionally exposed to infective products to cause disease at a stage and level at which overall health is not compromised. Exposed animals are expected to survive and develop immunity.
Producers purchase enough disease-free replacement gilts for four to six months, and keep them separate from the current herd. The herd is then closed and monitored for acutely ill piglets. Once scours are noted in affected piglets, one or more piglets are euthanised within six hours of the onset of scours. The piglets’ intestines are then macerated and washed with cold water. This material is added to the feed and fed to sows at the beginning or end of feeding periods. The infective dose must be high enough to cause clinical signs in sows, and intestines and faeces from piglets carry a higher viral load than those from adult pigs or dead piglets. Eventually, all exposed animals will exhibit clinical signs.
Each sow should be monitored daily for overt clinical signs (vomiting or diarrhoea) or more subtle clinical signs, such as decreased food intake or increased body temperature. Affected sows are marked clearly so that healthy animals can be identified. After the first few days of initial feedback, healthy animals can be fed infected material again until clinical signs are observed. To preserve infected material, intestinal viscera from the first feedback procedure can be frozen.
This process can also be performed in nursery, growing and finishing pigs to eliminate disease from the entire herd but additional care is needed to disinfect buildings, as contamination is more widespread within these groups of pigs. After the entire herd has been exposed and clinical signs have ceased, all-in/all-out movement of animals should be enforced. Barns should be thoroughly disinfected.

Sentinel animals are used to assess whether PEDv is present in the herd. These pigs are procured from the same negative source as the replacement gilts, and are confirmed negative for antibodies to PEDv. They are isolated for 30 days before being introduced into the herd. Sentinel pigs are observed daily for signs of enteric disease. If diarrhoea occurs in sentinel animals, they are euthanized and their tissues submitted for diagnosis.
Additional exposure of the herd to feedback material may be needed if sentinel animals are infected with PEDv. Blood is collected from the sentinels monthly to assess whether they have been exposed to the virus. If no clinical signs are observed in the sentinel pigs after 30 days of introduction, and their serology is negative for PEDv antibodies, the virus is considered eliminated from the herd.
Producers use feedback to mitigate disease impact in the absence of a reliable vaccine. Feedback is not recommended in herds affected with porcine reproductive and respiratory syndrome virus, as this can cause immunosuppression and older pigs may not recover from a co-infection with PEDv. Feedback is time-consuming and expensive, and the procedure is not ideal for producers who cannot enforce biosecurity or all-in/all-out systems or for low-production herds in some developing countries.
Is PEDv Reportable?
On 5 June 2014, the United States Department of Agriculture (USDA) issued a Federal Order requiring the reporting of PEDv and porcine delta coronavirus (PDCov) (reported as swine enteric coronavirus [SECov]) to the USDA Animal and Plant Health Inspection Service (APHIS) (USDA, 2014e). Canada requires reporting in some provinces.
OIE does not currently require reporting of PEDv but encourages it because of PED’s status as an emerging disease. Canada, the Dominican Republic, Mexico, the Republic of Korea, the US and others have reported PEDv to OIE.
References
AASV. 2013. PED update and biosecurity suggestions. American Association of Swine Veterinarians (AASV). Available at: http://www.aasv.org/aasv%20website/ Resources/Diseases/PED/PEDVBiosecurity. pdf (accessed 20 May 2014)
AASV. 2014. Porcine epidemic diarrhea virus (PEDv) testing data from NAHLN Laboratories. American Association of Swine Veterinarians (AASV). Available at: http:// www.aasv.org/pedv/PEDV_weekly_report_ 140129.pdf (accessed 20 May 2014)
Ackerman, M. 2013. Practitioner perspective when faced with PEDv. Available at: www.aasv.org/members/only/ PED/20130606PEDVAckerman.pdf (PowerPoint presentation)
Alonso, C., Goede, D.P., Morrison, R.B., Davies, P.R., Rovira, A., Marthaler, D.G. & Torremorell, M. 2014. Evidence of infectivity of airborne porcine epidemic diarrhea virus and detection of airborne viral RNA at long distances from infected herds. Vet. Res., 45(1): 73.
Bi, J., Zeng, S., Xiao, S., Chen, H. & Fang, L. 2012. Complete genome sequence of porcine epidemic diarrhea virus strain AJ1102 from a suckling piglet with acute diarrhea in China. J. Virol., 86(19): 10910–10911.
Alonso, C., Goede, D.P., Morrison, R.B., Davies, P.R., Rovira, A., Marthaler, D.G. & Torremorell, M. 2014. Evidence of infectivity of airborne porcine epidemic diarrhea virus and detection of airborne viral RNA at long distances from infected herds. Vet. Res., 45(1): 73.
Campbell, E. & Chen, L. 2014. Virus killing 5 million pigs spurs hog-price rally: commodities, Bloomberg News 6 February 2014. Available at: http://www. bloomberg.com/news/2014-02-06/ virus-killing-5-million-pigs-spurshog- price-rally-commodities.html (accessed 20 May 2014)
Carvajal, A., Lanza, I., Diego, R., Rubio, P. & Cármenes, P. 1995. Seroprevalence of porcine epidemic diarrhea virus infection among different types of breeding swine farms in Spain. Prev. Vet. Med., 23: 33–40.
CFIA. 2014. Porcine epidemic diarrhea (PED) situation in Canada. Canadian Food Inspection Agency (CFIA) Animal Disease Updates. Available at: http://www.inspection. gc.ca/animals/terrestrial-animals/ diseases/other-diseases/ped/ eng/1392762503272/1392762576176 (accessed 20 May 2014)
Chen, J., Wang, C., Shi, H., Qiu, H., Liu, S., Chen, X., Zhang, Z. & Feng, L. 2010. Molecular epidemiology of porcine epidemic diarrhea virus in China. Arch. Virol., 155: 1471–1476.
Daily Livestock Report. 2013. Mexico bans U.S. pig exports due to PED virus, 26 June 2013. Available at: http://nationalhogfarmer. com/health/mexico-bansus- pig-exports-due-ped-virus (accessed 20 May 2014)
Food Safety News. 2014. Virus scare causes Russia to suspend U.S. pig imports, 3 June 2014. Available at: http://www. foodsafetynews.com/2014/06/virusscare- causes-russia-to-suspend-u-spig- imports/#.U9PbVvldWSq (accessed 29 July 2014)
Geiger, J.O. & Connor, J.F. 2013. Porcine epidemic diarrhea, diagnosis, and elimination. Available at: http://www.aasv. org/aasv%20website/Resources/Diseases/ PED/13-05-29PEDWhitePaper. pdf (accessed 20 May 2014)
Goyal, S. 2014. Environmental stability of PEDV. AASV PEDV Research Updates (preliminary results), 21 January 2014. Available at: http://www.aasv.org/pedv/ research/13_215.pdf (accessed 20 May 2014)
Harris Vaccines. 2014. Porcine epidemic diarrhea virus. Available at: http://www.harrisvaccines.com/en/products/ porcine_epidemic_diarrhea_virus/ (accessed 29 July 2014)
Hesse, D. 2013. Tissue localization, shedding, virus carriage, antibody response, and aerosol transmission of porcine epidemic diarrhea virus (PEDV) following inoculation of feeder pigs. AASV PEDV Research Updates (preliminary results), 25 November 2013. Available at: http:// www.aasv.org/pedv/research/13_228. pdf (accessed 20 May 2014)
Huang, Y., Dickerman, A.W., Piñeyro, P., Li, L., Fang, L., Kiehne, R., Opriessnig, T. & Meng, X.-J. 2013. Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. Molec. Bio., 4(5): 737–813.
Kim, O. & Chae, C. 2000. In situ hybridization for the detection and localization of porcine epidemic diarrhea virus in the intestinal tissues from naturally infected piglets. Vet. Pathol., 37(1): 62–67.
Li, Z.L., Zhu, L., Ma, J.Y., Zhou, Q.F., Song, Y.H., Sun, B.L., Chen, R.A., Xie, Q.M. & Bee, Y.Z. 2012a. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea virus (PEDV) field strains in south China. Virus Genes, 45(1): 181–185.
Li, W., Li, H., Liu, Y., Pan, Y., Deng, F., Song, Y., Tang, X. & He, Q. 2012b. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg. Inf. Dis., 18(8): 1350–1353.
Martelli, P., Lavazza, A., Nigrelli, A.D., Merialdi, G., Alborali, L.G. & Pensaert, M.B. 2008. Epidemic of diarrhoea caused by porcine epidemic diarrhoea virus in Italy. Vet. Record, 162: 307–310.
Meyer, S. 2013. How is porcine epidemic diarrhea (PED) virus impacting hog markets? Available at: http://nationalhogfarmer. com/business/how-porcine- epidemic-diarrhea-ped-virus-impacting- hog-markets (accessed 20 May 2014)
Pan, Y., Tian, X., Li, W., Zhou, Q., Wang, D., Bi, Y., Chen, F. & Song, Y. 2012. Isolation and characterization of a variant porcine epidemic diarrhea virus in China. Virology J., 9: 195–204.
Puranaveja, S., Poolperm, P., Lertwatcharasarakul, P., Kesdaengsakonwut, S., Boonsoongnern, A., Urairong, K., Kitikoon, P., Choojai, P., Kedkovid, R., Teankum, K. & Thanawongnuwech, R. 2009. Chinese-like strain of porcine epidemic diarrhea virus, Thailand. Emerg. Inf. Dis., 15(7): 1112–1115.
Schwartz, K., Henry, S., Tokach, L., Potter, M., Davidson, D. & Egnor, C. 2013. Infective material, concepts and procedures for intentional sow herd exposure to porcine epidemic diarrhea virus. Available at: http://www.aasv.org/pedv/Conceptsforherdexposure121713.pdf (accessed 20 May 2014)
Song, D. & Park, B. 2012. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes, 44: 167–175.
Sun, R.Q., Cai, R.J., Chen, Y.Q., Liang, P.S., Chen, D.K. & Song, C.X. 2012. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg. Inf. Dis., 18(1): 161–163.
The Pig Site. 2013. China: hog markets. Available at: http://www.thepigsite.com/ swinenews/34464/china-hog-markets (accessed 20 May 2014)
USDA. 2014a. Swine Health Monitoring Project, 10 January 2014, PEDV Swine Disease Eradication Center. United States Department of Agriculture (USDA). Available at: http://www.cvm.umn.edu/ sdec/prod/groups/cvm/@pub/@cvm/@ sdec/documents/content/cvm_content_ 468272.pdf (accessed 20 May 2014)
Reference
Hill, C., Raizman, E., Snider, T., Goyal, S., Torremorell, M. and Perez, A.M. 2014. Emergence of porcine epidemic diarrhoea in North America. FOCUS ON, No. 9, July 2014. Rome. Original source: FAO report - www.fao.org/3/a-i3967e.pdf.
August 2014






