



African swine fever in wild boar: infection routes and mechanisms
ASF infection routes - route of introduction and transmission chainEditor's note: the following is an excerpt from African swine fever in wild boar populations - ecology and biosecurity. It was created by the FAO, WOAH and European Commission. Additional content from the booklet will be shared as an article series.
Direct horizontal transmission: The usual physical contact among wild boar in the same group, and sometimes, with individuals from other groups, is sufficient to transmit the virus, as happens with many other infectious diseases. Direct horizontal transmission plays a very important role in habitats with relatively high wild boar density, as happens when the virus is newly introduced into a disease-free population.
Local indirect transmission through contaminated environment: The habitats where the infected wild boar population lives can be heavily contaminated through the excretions and secretions of infected individuals and their remnants after death (that is, whole carcasses or parts disseminated by scavengers), and from infected materials originating from the hunting of ASF-positive animals (blood, meat and offal) that spill into or are disposed of directly into the habitats. The effectivity of environmental transmission is impacted by the time of year, the weather and other factors.
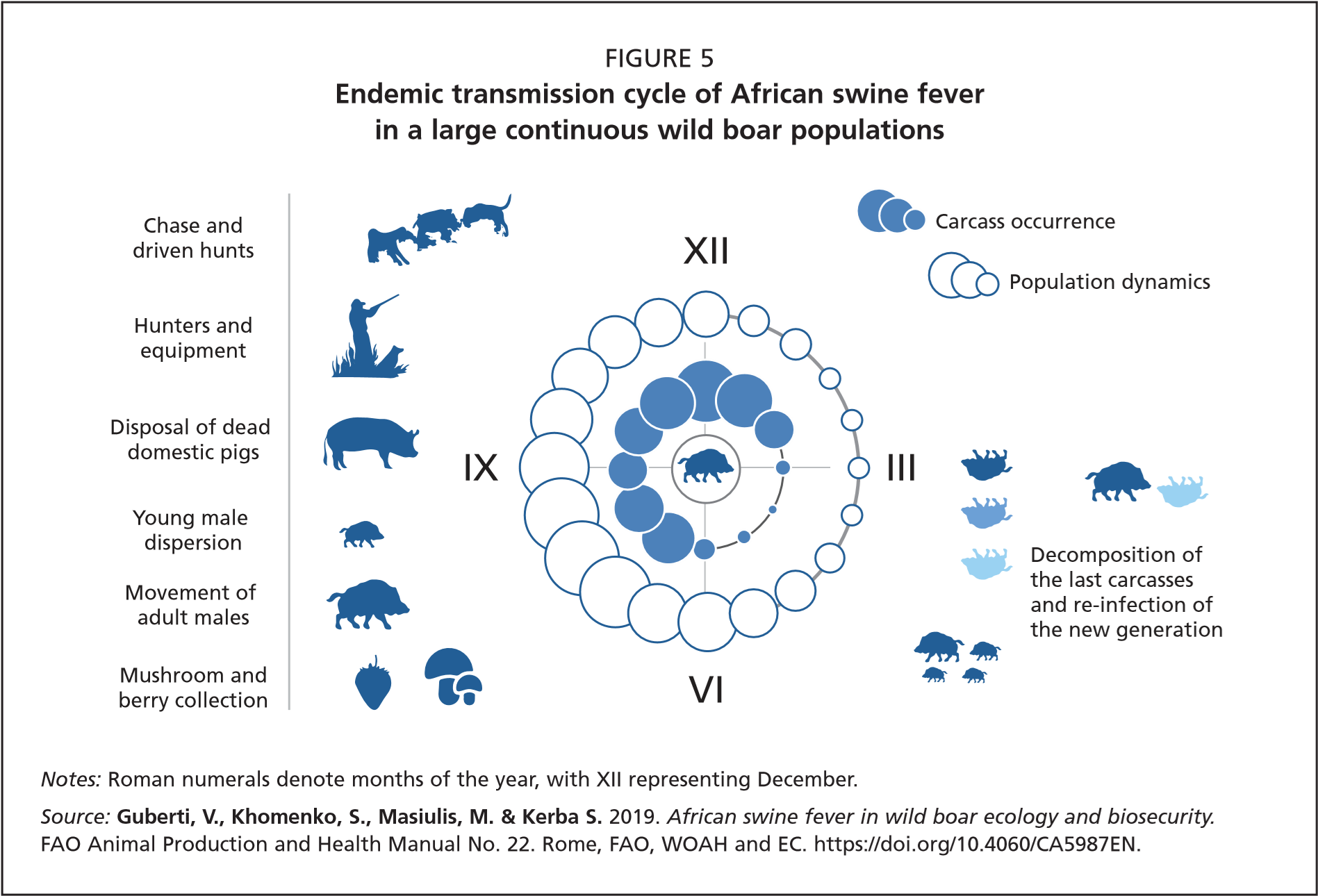
Infected carcasses: The indirect transmission via infected carcasses of wild boar (or domestic pigs) is considered to play a pivotal role in the epidemiology of ASF (see Box 2 for the results of the first study into the topic). Infectious carcasses have the capacity to maintain live virus in the habitat for a much longer period of time (months) compared to its persistence in excretions, especially during winter, thus making wild boar population density and contact rates less relevant for long-term maintenance of the ASF transmission cycle. Particularly in summer, after they pass through the first stages of decomposition, these carcasses provide good conditions for the development of communities of invertebrate insects that can further attract susceptible, healthy wild boar.
Remnants of infected animals: Offal abandoned by hunters when dressing infected animals in the field may also play a relevant role by increasing virus loads in the environment. A susceptible wild boar living in a habitat contaminated in this way has a high probability of becoming infected with the virus.
Excretions: The virus excreted with urine and faeces contaminates wild boar habitats and, during periods when temperatures are low (especially winter) which are favourable to the survival of the virus, can be transmitted to susceptible animals. In proximity to wild boar feeding points, environmental contamination may be of high importance. In winter, provided with regular supplementary feeding, some wild boar tend to reduce the extent of their home range to within just 200–300 m of the feeding point, while other individuals regularly visit all the feeding points, acting as a connector among feeding sites. This tendency, along with the increased probability of encountering other individuals that can spread infection through direct horizontal transmission, also increases probability of infection. Excretions may also contaminate crops and grass, which can subsequently end up as fresh feed in domestic pig stables.
Risk of Introduction
ASF is introduced to new wild boar populations through two main mechanisms summarized below and in Table 1. The first is human-mediated or anthropogenic introduction, in which people carry the virus from infected into free areas over long, medium or short distances. Such unintended translocations of the disease may involve anybody who has been in contact with the infection or carries contaminated products of pig origin, including groups such as hunters, mushroom collectors, tourists, the military, loggers and farmers. People can transport the virus over long distances through contaminated meat and other products such as skins, skulls, tusks and other hunting trophies. Irrespective of whether the virus originates from domestic pigs or wild boar, and even if unintended or accidental, this mechanism provides the means of spreading the disease over distances greatly exceeding those involved with the transmission mechanisms already described.
Release of the virus by humans through contaminated materials is particularly dangerous because the disease may flare up in unexpected locations far from known outbreaks in domestic pigs or cases in wild boar. On multiple occasions, including in Europe, indirect long-distance spread of the virus has initiated new clusters of infection in wild boar (and in domestic pigs), some of which have now developed into long-lasting epidemics, as mapped in Figure 3. The most recent examples are the localized epidemics of ASF in Czechia (Zlin District), Poland (Warsaw and western Poland), Hungary (Heves County) and Belgium (Étalle). According to EFSA (2018), anthropogenic introduction has played a pivotal role in the epidemiology of ASF in the wild boar populations of northern and eastern Europe; the anthropogenic introduction and spread of ASFV among wild boar populations is a continual risk which is difficult to minimize.
The second main mechanism of introduction is through an unfolding epidemic, which expands the virus circulation due to the geographical continuity of the infected wild boar population. It is the most common way in which the virus progressively spreads across the landscape, affecting adjacent host populations in new areas because of their spatial and ecological continuity with already infected areas. Unless inappropriate control measures are applied on the geographical edges of the epidemic (such as, for example, driven hunts which strongly enhance disease progression through increasing the mobility of animals), this type of ASF spread is a purely natural process and does not involve any human action. It is characteristic for a naturally progressing epidemic to initially produce multiple introductions in new areas, as was the case with the ASF epidemic crossing the border between Poland and Germany. This type of introduction is highly predictable, although almost unavoidable without efficient barriers. It becomes a real challenge to ensure effective surveillance and control in the newly infected areas when the virus arrives in a frontal fashion.
Transmission chain in wild boar populations
Once the virus is introduced into an area, an epidemic is likely to occur and evolve in a fairly predictable manner. The epidemiological cycle of ASF in wild boar is characterized by a combination of a simultaneous steady geographic spread (epidemic wave) to neighbouring disease-free areas, and local endemic persistence. These elements are now explored in greater detail:
Epidemic wave: Initially, the virus spreads as a wave, leaving multiple ASF-positive carcasses in its wake. The speed (velocity) of the wave depends on the density of the wild boar population: the higher the density, the faster the velocity at which the epidemic moves. The velocity can range from approximately 1 km/month to 1 km/week, according to wild boar density and human activities such as hunting. In Belgium, the speed was higher due to the higher wild boar density. Calculations show that natural geographical spread of ASF in areas of typical density of wild boar populations for northern and eastern Europe occurs at the speed of several kilometres per month, resulting in continuous expansion of the epidemic wave, followed by local endemic persistence (EFSA, 2017; Belgium data). Differences in the speed of the infection may also be impacted by the timing of incursions, the continuity of suitable wild boar habitat and the types of interventions and management activities put in place.
Wild boar are generally a sedentary species (Podgórski et al., 2013), with stable group home ranges rarely exceeding 50 km2. However, episodes of long-distance spread of the virus significantly beyond the normal movement range of wild boar have occasionally been observed. There have been incidences of some significantly long-distance movements, for example approximately 100 km in six months (Jerina et al., 2014). Possible longer-range movements during which an infectious (i.e. in incubating and disease phases) animal might spread the virus (e.g. young males during dispersion period or adult males in pursuit of females in heat) last for a limited time, roughly 5–7 days. In the course of a week, wild boar (particularly when undisturbed and sick) are highly unlikely to cross large distances. Hence, long-range incursions of ASF are most likely caused by human activities, although their unintended or illegal nature (often accompanied by a lack of awareness of the sources of the virus and its transmission mechanisms) make this difficult to prove with sufficient epidemiological evidence.
The direction of the wave is determined by the suitability of wild boar habitats. Large coterminous forested areas and/or wetlands will facilitate the spread of the virus. The speed of the virus wave is lower in fragmented habitats and where natural or artificial barriers disrupt habitat continuity. Artificial or natural barriers rarely halt the epidemic wave, but they do slow its speed, making it possible for the authorities to plan and implement specific disease control interventions. In the absence of entirely effective barriers, it is only a matter of time before the virus can spread indefinitely, reaching any wild boar (meta)population in geographical continuity with the infected one(s).
Direct animal-to-animal transmission of the virus is prevalent at the onset of the infection, during the epidemic phase. Intensification of direct transmission may also occur following the reproductive season, when the host population size almost doubles and newborn individuals (from 2 to 6 months of age) explore their habitat. This behaviour increases intraspecific contact, as does the regrouping or aggregation of herds when it occurs in cereal fields or protected reserves. At the peak of the unfolding epidemic, human interventions can neither prevent nor substantially enhance the demographic crash of the ASF-infected population; mortality rates produced by the disease significantly exceed human capacity to react to this crisis (Morelle et al., 2020).
Endemic persistence: The more effective the spread of the virus, the sooner it will lead to a relatively rapid decline of the wild boar population. Ultimately, as a result of decreasing populations, intraspecific contact also declines and the epidemic moves into an endemic phase. Following the initial epidemic wave, the virus remains endemic in all those areas crossed by the wave. With the decline of wild boar abundance, the indirect mode of transmission through infectious carcasses and/or contaminated habitat becomes more important, favouring the local maintenance of endemic infection. Robust relationships between low density and indirect transmission of the virus have been highlighted in the endemic areas of Poland and are further shown in modelling (Pepin et al., 2020; Gervasi and Guberti, 2021).
The endemicity of ASF in wild boar can be sustained naturally; however, the natural disease dynamics are often intermingled with human actions propagating the virus. Some counteractive hunting practices, including human attendance at feeding locations, disposal of contaminated offal and the involvement of fomites are among the most frequent faults contributing to disease endemicity. Finally, the presence of infected domestic pigs, and the illegal disposal of their carcasses in the environment where wild boar may come in contact with them, will further increase the probability of virus persistence. In such an epidemiological landscape, the interference consisting of short-, medium- or long-distance anthropogenic introductions of the virus make the complete eradication of ASFV extremely unlikely.
The complexity of the spatial dynamic: The typical chain of events associated with the introduction of ASF to a new area is illustrated in Figure 6. The index case (incursion), which is almost always missed by scientists and the authorities who monitor the status of ASF in their national or regional territories, creates a successful invasion that, on most occasions, also remains a “silent” epidemiological event which fails to trigger any control interventions. The invasion then turns into an apparent epidemic wave; only now is opportunistic passive surveillance likely to detect the worrisome signals. In the areas impacted by the epidemic, the infection remains endemic for extended periods of time. As the size of this endemic zone grows, the probability of further anthropogenic translocations of the virus and introductions into virus-free areas increases. The new introductions lead to new successful invasions, evolving into another epidemic wave. Expanding waves tend to merge, resulting in enlarged areas where the virus persists endemically; without specific control interventions, the cycle is repeated again and again. Such a pattern of disease activity is also fuelled by the re-establishment of the wild boar population following the initial demographic collapse, and recolonization of the territories where animals were killed by the disease.
Guberti, V., Khomenko, S., Masiulis, M. & Kerba S. 2022. African swine fever in wild boar – Ecology and biosecurity. Second edition. FAO Animal Production and Health Manual No. 28. Rome, FAO, World Organisation for Animal Health and European Commission. https://doi.org/10.4060/cc0785













