



African swine fever in wild boar: epidemiology
Epidemiological cycles and geographical distribution of ASF in EuropePart of Series:
Next Article in Series >
Editor's note: the following is an excerpt from African swine fever in wild boar populations - ecology and biosecurity. It was created by the FAO, WOAH and European Commission. Additional content from the booklet will be shared as an article series.
ASF is a disease of pigs, which was originally associated with the ecological niche of the ticks of the genus Ornithodoros and the common warthog (Phacochoerus africanus) in sub-Saharan Africa. Warthogs and ticks, which naturally co-inhabit burrows, can sustain the transmission cycle of this virus for an unlimited time. It is a well-established natural host–vector–pathogen system, the sylvatic transmission cycle of ASF (Penrith and Vosloo, 2009), with a distribution restricted to parts of the African continent. Warthogs are naturally resistant to the effects of the African swine fever virus (ASFV) and do not usually develop clinical disease. Infection takes place within burrows, where a strong symbiotic relation exists between the warthogs and ticks . Young warthogs are born uninfected, and first become infected when bitten by O. moubata. They then develop viraemia which lasts for two or three weeks, which is sufficient to infect new ticks in turn (Thomson et al., 1980).
In Africa, the virus has shown a trend to shift towards a more anthropogenic cycle (Figure 1,cycle 2) in which domestic pigs instead of warthogs assumed the role of an epidemiological reservoir, with the occasional involvement of Ornithodoros ticks. This kind of transmission cycle was also reported from the Iberian Peninsula during the 1960s and 1970s (Sánchez-Vizcaíno, Mur and Martínez-López, 2012). Again, in Africa, driven by the growing human population and increasing numbers of domestic pigs, ASF spread to areas where it had never occurred naturally before. In these new areas, its transmission cycle no longer involves ticks or warthogs (Figure 1, cycle 3). The spread of the virus in domestic pigs is facilitated by human activity. Movements of animals due to trade, the sale of infected meat and free-range pig farming are the main risk factors in this system. A similar, purely domestic, pig cycle, has also evolved in the Caucasus since 2007 (European Food Safety Authority [EFSA], 2010a, 2015), when the genotype II virus was first introduced in Georgia. Thereafter, it has spread northwards, primarily in the domestic pig population, moving from the Caucasian countries to the Russian Federation, Belarus and Ukraine, and then to other European countries (Gogin et al., 2013; Figure 2 and Figure 3).
Finally, the most recent step in the evolution of the biological cycle of ASFV and its geographical spread is related to the formation of the wild boar–habitat cycle (Figure 1, cycle 4) which has developed in northern and eastern Europe. This novel host–pathogen–environment system has steadily expanded the range of ASF in Europe (EFSA, 2017), facilitated by the exceptional stability and resilience of ASFV in the environment and infected carcasses of animals. Since 2014, spread has occurred in the Baltic states, Poland and Czechia (Khomenko et al., 2013; EFSA, 2017), followed by Hungary, Romania and Belgium, and later Slovakia, Greece and Germany, with Italy and North Macedonia being the most recent (January 2022) European countries to be affected.

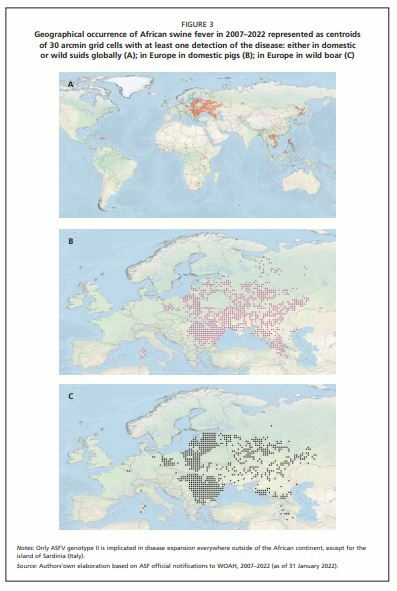
This cycle is characterized by the continuous presence of the virus in the affected wild boar populations, which represents a serious challenge for the pig production sector and wildlife management authorities, as well as hunters. In the last four years, ASF has become endemic in wild boar over remarkably large areas (Figure 3), and the scale of the problem poses a major threat to the European pig production sector (Figure 2). From 2018 onwards, genotype II ASFV spread eastwards into Asia, affecting China, Hong Kong, the Democratic People’s Republic of Korea, the Republic of Korea, the Lao People’s Democratic Republic, Viet Nam, Myanmar, Cambodia, Indonesia, Philippines, Timor-Leste, Papua New Guinea and India (Penrith, 2020), crossing the Atlantic to be detected in the Dominican Republic and Haiti in 2021 WOAH, 2021); in some Asian countries, wild boar populations seem to be taking the same role which has been observed in Europe. This chapter is mainly based on knowledge accumulated in the last decade on the epidemiology of ASF genotype II in the wild boar in central and eastern Europe, although reference is occasionally made to specific epidemiological situations outside this area.
The ongoing progressive spread of ASF around the world opens possibilities for formation of new, as yet undescribed, epidemiological cycles of ASF. These might involve domestic, feral or wild suids, with or without involvement of competent or mechanical vectors of the virus. Naturally or artificially attenuated strains of ASF have a potential to change the epidemiology of ASF, resulting in host–pathogen–environment constellations that are difficult to predict. Given the exceptionally wide distribution of susceptible host species of the genus Sus, the ASF clearly has panzootic potential, and if not promptly controlled, might become endemic on any continent (except Antarctica) shortly upon incursion.
Characteristics of the ASF virus circulating in Europe and Asia
ASF is caused by a DNA virus belonging to the Asfarviridae family. It affects species belonging to the Suidae family only; in Europe, the sole susceptible species are domestic pigs and wild boar (Sus scrofa). They show similar clinical signs and have similar case fatality rates. Although a total of 24 genotypes of the virus are known to circulate in Africa, only two currently occur in Europe, both in wild boar and domestic pigs (Gabriel et al., 2011). Genotype II spread extensively in eastern Europe from 2007, while genotype I had been reported as endemic in Sardinia only, where it remains confined and is under systematic observation and control (Franzoni et al., 2020). The genotype II virus now circulating in Europe and Asia has a very high case fatality rate of over 90 percent, irrespective of whether the infected animals are wild or domestic.
Being a DNA virus, the genetic structure of ASFV is rather stable, and thus the use of molecular epidemiology for tracing back the origin of the virus is of limited use, though a few mutated and attenuated Georgian genotype II viruses have been isolated in Europe (Zaniet al., 2018; Gallardo et al., 2019). However, recent findings from Poland and Germany demonstrate the existence of new variants facilitating molecular epidemiological tracing (Mazur-Panasiuk, WoǍniakowski and Niemczuk, 2019).
The attenuated strains of the Caucasian genotype II seem to show milder clinical symptoms and a reduced lethality. Under the field conditions, the attenuated strains should – at least in theory – cause higher seroprevalence, and have a higher probability of locally persisting as an endemic infection. The spread of the attenuated strains would pose a change in the early detection strategy, which would then not be solely based on passive 8 African swine fever in wild boar: ecology and biosecurity – Second edition surveillance. So far these attenuated strains have failed to replace the original highly lethal strain. For instance, the Estonian strain has already disappeared, while the Latvian strain can be still isolated, but its prevalence is extremely low. With carcasses being the most important source of virus contamination of the environment and, in this way, for infecting susceptible wild boar, in most European countries the virulent ASFV strains of genotype II do not so far seem to be losing their adaptive advantage by retaining their lethality. Counterintuitively, if the host mortality contributes to the spreading of the virus, which is apparently the case with ASF in wild boar, the highly virulent strains are naturally selected for continued transmission. The behaviour of an attenuated strain when spreading into a wild boar population is hard to predict, but currently it seems to be the virus lethality that helps it to propagate itself in the wild boar–habitat cycle. For low-virulence strains to become established, their transmission rate would have to increase, which has not been observed so far.
Reference
Guberti, V., Khomenko, S., Masiulis, M. & Kerba S. 2022. African swine fever in wild boar – Ecology and biosecurity. Second edition. FAO Animal Production and Health Manual No. 28. Rome, FAO, World Organisation for Animal Health and European Commission. https://doi.org/10.4060/cc0785













