



African swine fever in wild boar: population density
ASF dynamics and wild boar population densityEditor's note: the following is an excerpt from African swine fever in wild boar populations - ecology and biosecurity. It was created by the FAO, WOAH and European Commission. Additional content from the booklet will be shared as an article series.
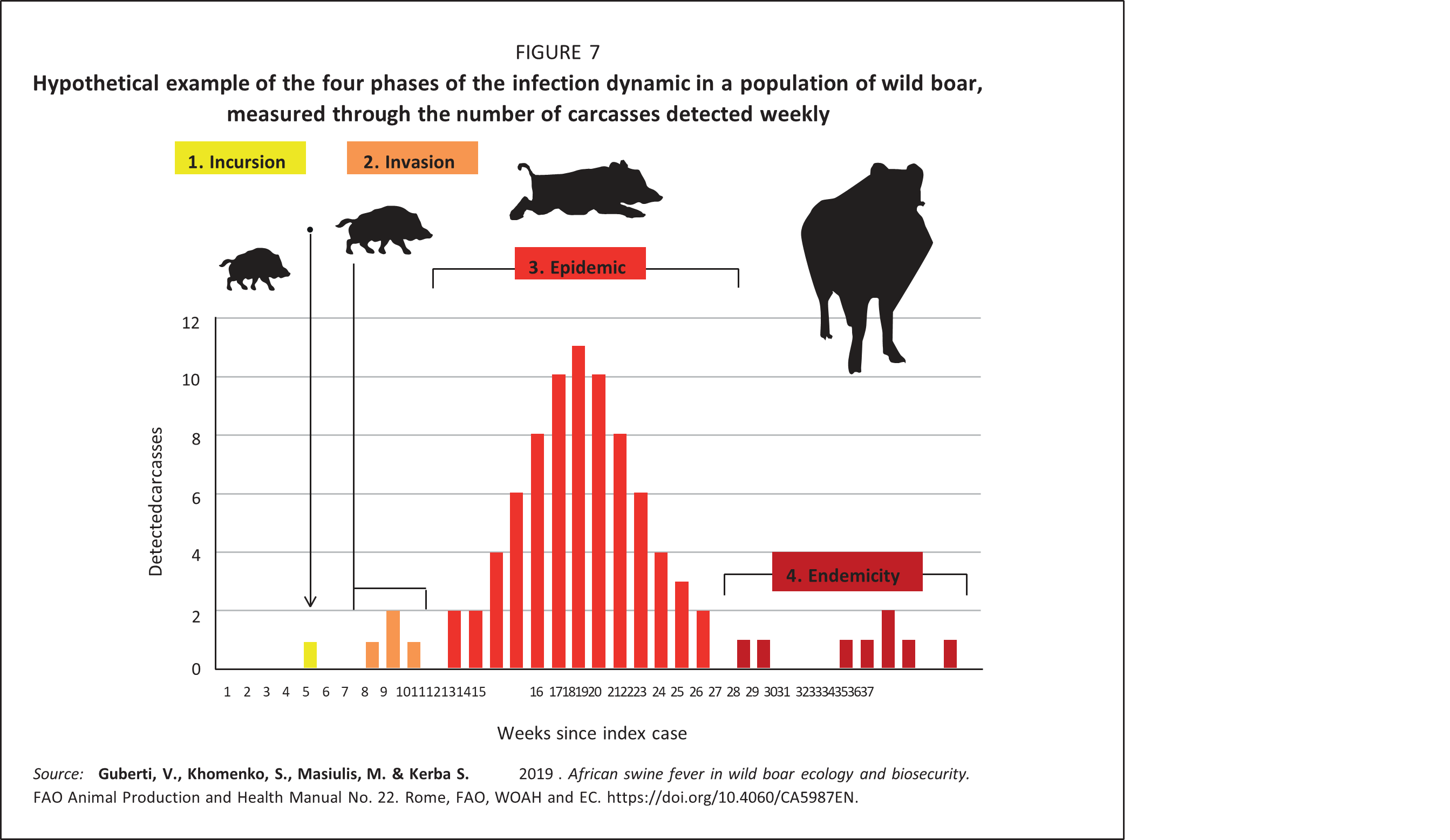
Understanding the relationship between the spread of ASFV and the wild boar population density is of paramount importance, since major efforts in controlling the infection are based on population density and size reduction. The natural history of infectious diseases (Burnet and White, 1972) highlights the quantitative relationship between a transmissible disease agent and the host population. Four main phases of the infection dynamics at the population level are recognized: introduction (or incursion), invasion, epidemic and endemic persistence (Figure 7).
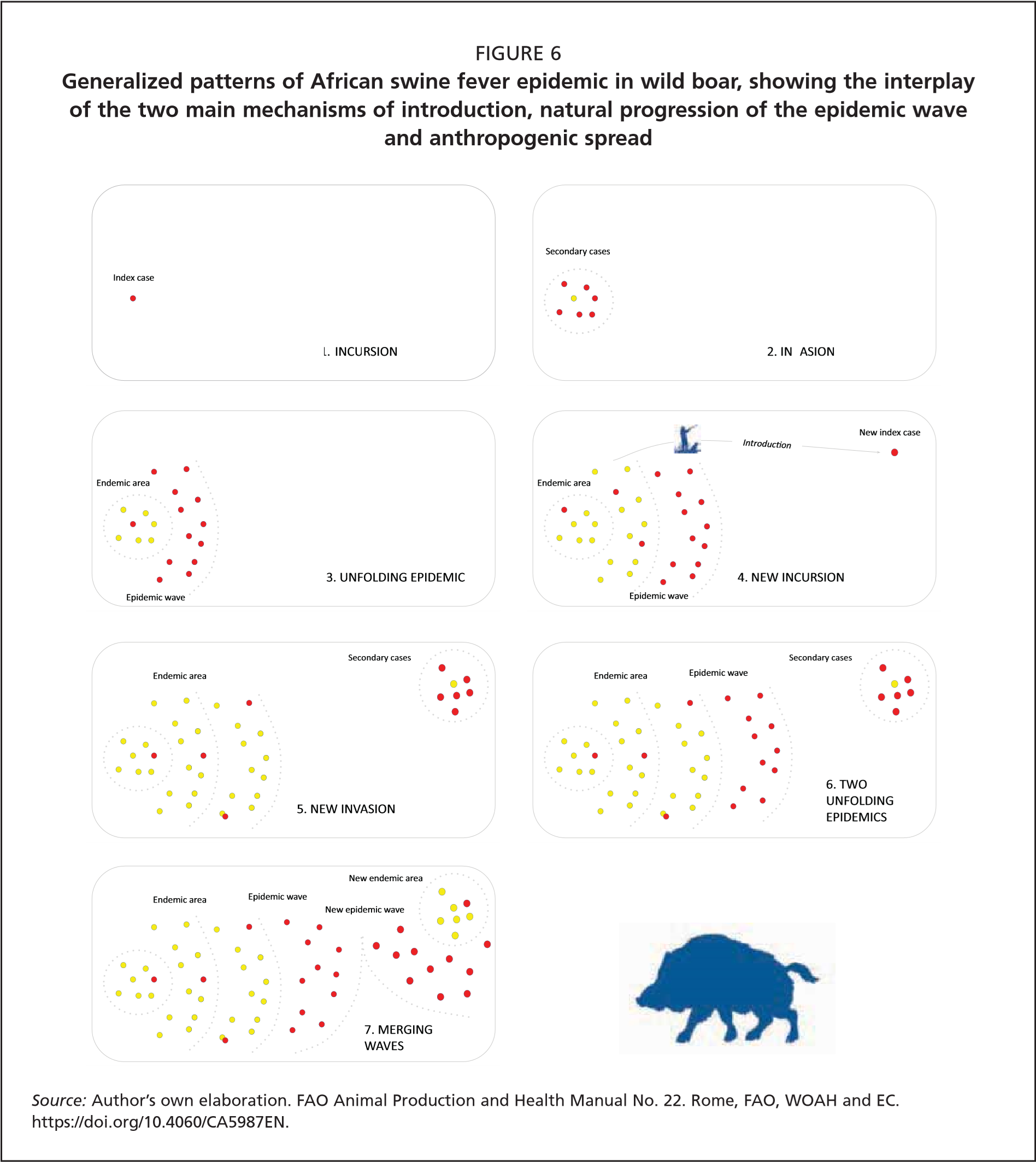
Incursion phase: This is the initial introduction of the virus into a disease-free, susceptible wild boar population. The incursion can happen through virus spread from a neighbouring infected wild boar population, or through accidental release of the virus in contaminated materials, often mediated by humans. The probability of the occurrence of an incursion is independent of the size and density of the local wild boar population.
Invasion phase: This is the initial successful spread of the virus in a susceptible wild boar population following an incursion. The probability that an infected wild boar will spread the virus depends on the availability of susceptible hosts. Any virus will spread when a large number of susceptible hosts are available. Conversely, in the absence of any susceptible hosts, the virus will become extinct, so the numbers and the density of available hosts will determine the outcome of the invasion (Figure 8).
For infections with a density-dependent dynamic, it is possible to estimate the minimum number of susceptible animals per unit of area needed to trigger a successful invasion. This number is called host threshold density, referred to as Nt. Nt-1 is the density at which an infectious host fails to encounter any susceptible individual within the time frame for transmission of the infection (Anderson and May, 1991; Lloyd-Smith et al., 2005). It is important to underline that the Nt value is mainly determined by the virus characteristics. Its practical use is restricted to the initial spread of an infection (that is, the invasion phase) and not to epidemic or endemic situations (Deredec and Courchamp, 2003; Lloyd-Smith et al., 2005).
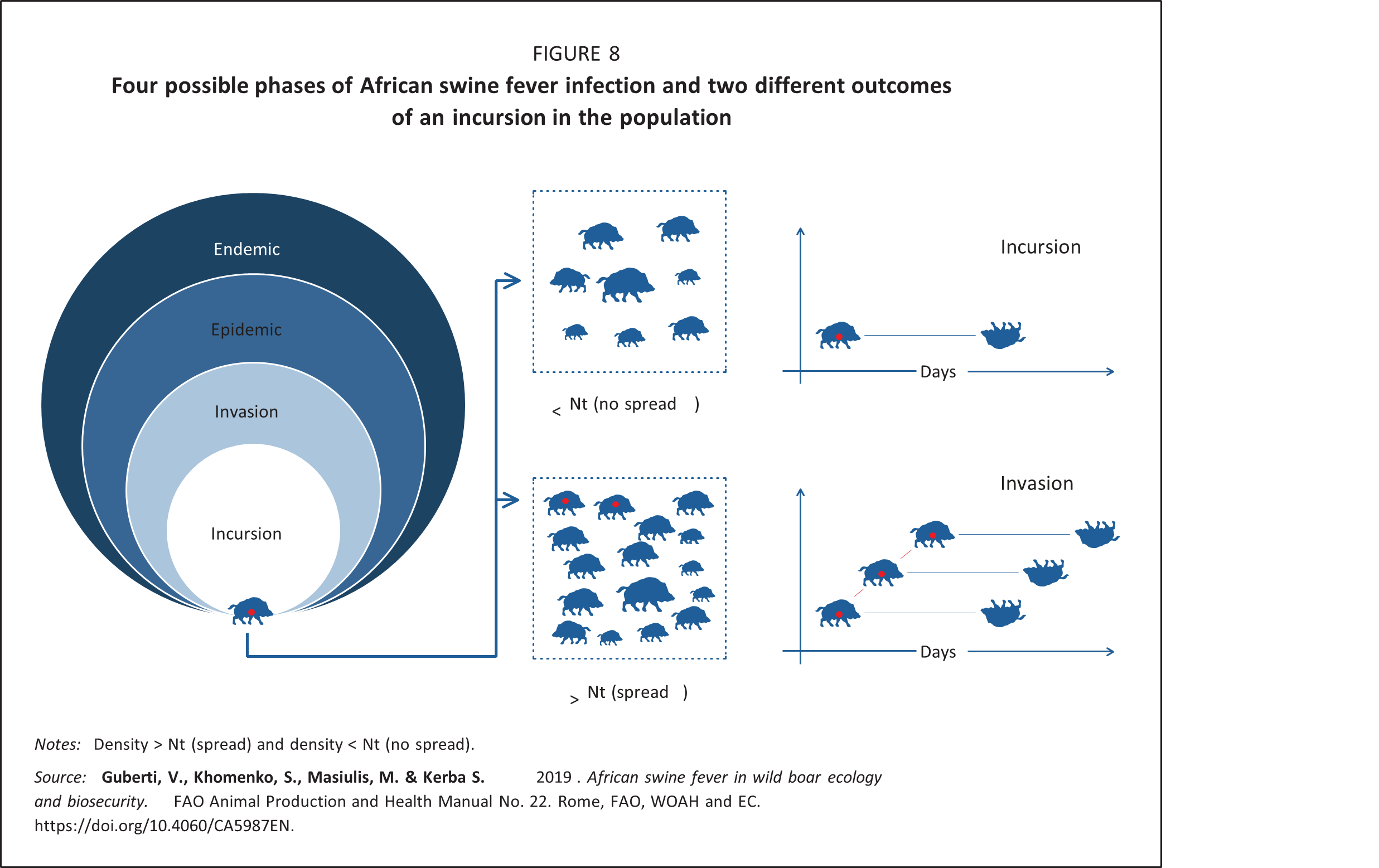
Among other methods used to control a disease, one might try to reduce the host population density to a level where the disease incursion would not be able to develop into an invasion and eventual epidemic. The Nt can be reached through depopulation or the direct elimination of all the animal categories, including those animals which are susceptible, infected and immune. Vaccination and immunization are also means of reducing the number of susceptible individuals though, unlike depopulation, the host population’s size and density will remain unaffected. In the case of ASF, no vaccine is currently available, so the only option is reduction of the population size and density.
The demographic parameters needed to estimate Nt are almost unknown, due to the lack of reliable estimates of wild boar population sizes for affected populations; appropriate data are available only for a few, ad hoc investigated populations, most of which are outside the range of ASF occurrence. In general, wild boar population size data are very poor, obtained using unstandardized methodologies with unknown error variability, and as such are mainly useful for describing trends rather than real population densities or sizes. As for the epidemiological data, they are collected in infected wild boar populations in which two different mixed transmission mechanisms, such as direct contact plus carcass-mediated infection, co-occur. It is a matter of fact that any mathematical estimation of Nt is simply impossible, most likely because a constant Nt does not exist for ASF in wild boar.
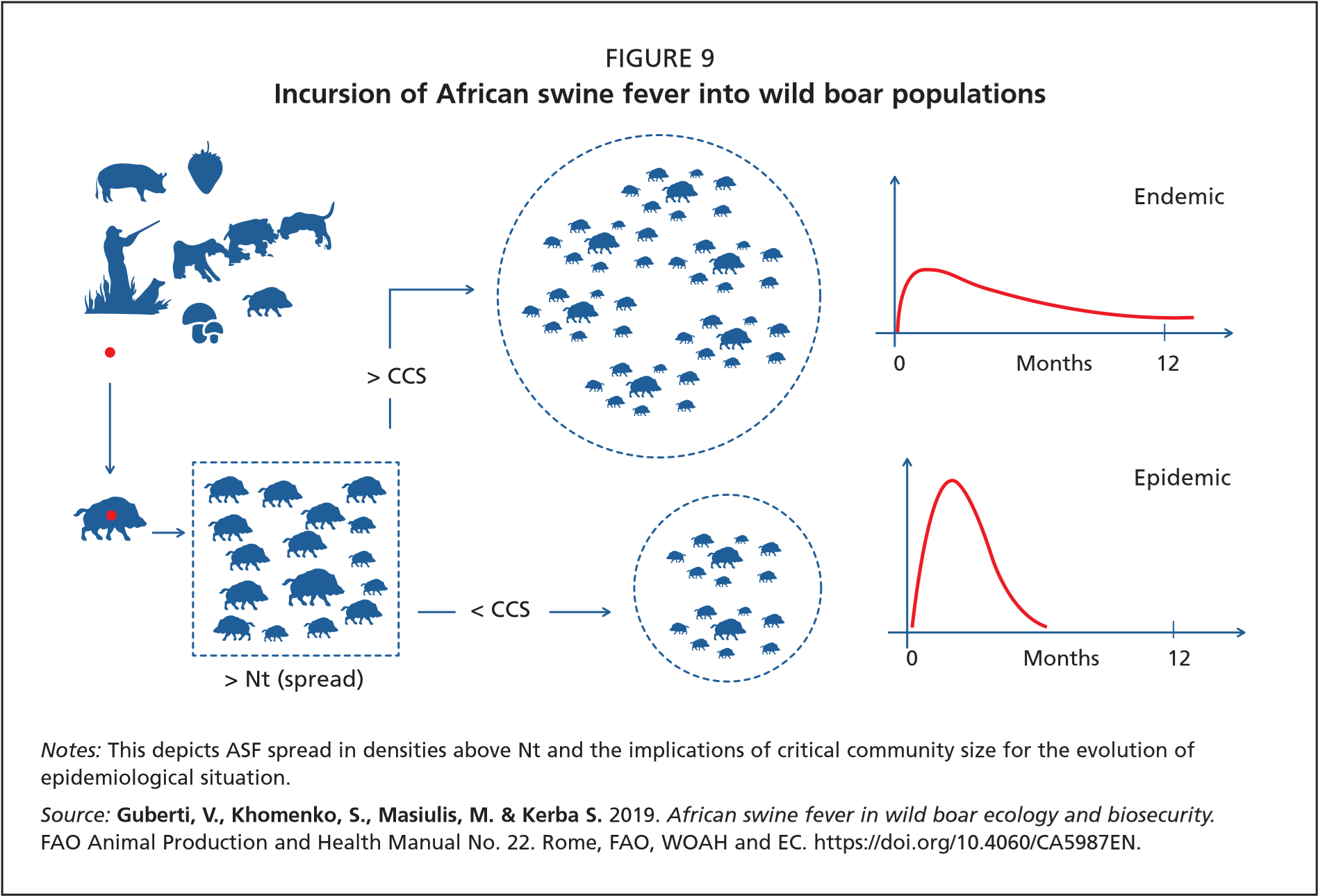
In any case, the practical application of the density threshold approach is justified in wild boar populations at risk of ASF as a preventive measure (i.e. in surrounding virus-free areas; see chapter 4). The logic behind using the Nt-oriented population management approach is that even if the virus incursion cannot be prevented, its further successful spread in the population with density below Nt will be unlikely because of insufficient numbers of susceptible wild boar.
Epidemic phase: This phase follows a successful invasion. The host population density is above Nt and thus the virus can spread and progressively invade the local wild boar population. The epidemic phase is described by a typical epidemic curve, the gradient and width of which depends on the quantitative relationship between the virus and the host populations. At high host density the epidemic curve is steep and narrow, while at the lower host density it is flat and wider. The number of contacts between infectious and susceptible animals drives the shape of the epidemic curve (Figure 9, graphs on right).
During the epidemic period, disease-independent mortality plays an important role in disease progression and can be used to modulate its outcome. Since the most common source of disease-independent mortality in wild boar is hunting, it is theoretically possible to modify the natural course of the infection by simply reducing the numbers and eventually the contact rate between susceptible and infectious wild boar by hunting them. The main effect of hunting is to accelerate the evolution of an epidemic into an endemic situation, which would naturally take longer to achieve (Swinton et al., 2002; Choisy and Rohani, 2006). However, in shaping a longer-lasting epidemic, the recruitment rate of new susceptible individuals through reproduction or immigration also plays a crucial role and should be accounted for. Failure to keep numbers below Nt may, again, result in a recurrent epidemic.
Managing ASF during the epidemic phase is a prohibitive task. At the onset of the epidemic, the number of infected individuals is higher than in any other phase, and any depopulation effort hardly matches the rate at which the virus spreads. The probability (p) of having a successful chain of ASF cases is determined by the number of infectious individuals (I) that are present in that specific time (t) according to p=1-(1/R0)It (Lloyd-Smith et al., 2005) where R0 is the number of secondary infections determined by each infected wild boar (Anderson and May, 1991; Marcon et al., 2019). During the epidemic phase, the probability of eradicating the infection is almost 0, due to the large number of infectious individuals. Moreover, since depopulation activities are not selective towards infectious animals (that is, not all infected animals are shot and removed from the hunting ground), they will die of the disease and, as infected carcasses, further contribute to the maintenance of the virus in the area. Both theoretical modelling and field evidence show that any intervention during the epidemic phase is likely to enhance those host population resilience mechanisms that facilitate infection persistence (Swinton et al., 2002; Choisy and Rohani, 2006).
Moreover, only a small percentage of carcasses (< 10 percent) are found and safely destroyed in most kinds of wild boar habitats (EFSA, 2015); thus, the virus is usually detected rather late, and usually during the epidemic period following a successful invasion. In practice, what is perceived as the invasion phase (e.g. the very first detection of an infected carcass) is, in reality, the onset, or sometimes even the peak, of a previously “silent” epidemic, with a large number of infected carcasses already present in the area. However, in the infected area, the number and timing of detected carcasses is the sole available tool for following the entire spread process, including identification of the different phases of the evolution of the infection.
Endemic phase: After the epidemic peak is passed, any disease either becomes endemic or fades out. Endemic evolution does not depend merely on host density (as described above concerning Nt), but also on the availability of a host critical community size (CCS). The CCS is defined as the minimum population size, rather than density, with which a pathogen has 50 percent probability of fading out spontaneously (Bailey, 1975; Nåsell, 2005).
The value of the CCS is variable for different pathogens and host species. In the case of ASF, it is mainly determined by wild boar biology and, in particular, by the main demographic characteristics of the population. A smaller CCS would sustain epidemics when the host population has a high turnover, short lifespan and high reproductive rates, which is the case for wild boar. The size of the CCS cannot be estimated using mathematical formulas, but can be obtained only through ad hoc computer simulations (McCallum, Barlow and Hone, 2001).
During the endemic phase, the ASFV spread and the wild boar population reach an equilibrium. Breaking this equilibrium through management interventions could be a way to make such populations unsuitable for sustained virus transmission, thereby eradicating ASF. However, multiple factors contribute to the endemic persistence of the infection, such as the real size of the wild boar population, the continuity of its distribution, population turnover and fertility, and thus the recruitment rate. The relative contribution of each factor to the endemic transmission cycle of ASF has not yet been properly evaluated. The strong contribution of the infected carcasses to the local maintenance of the disease cycle additionally complicates understanding of the whole dynamic of this novel host–pathogen–environment system. Intuitively, with the possible overwintering of the virus in infected carcasses, a simple depopulation approach aimed at reducing population density of animals is highly likely to fail to eradicate the disease. At a sufficiently low wild boar density (which is usually the aim of the depopulation efforts carried out during the epidemic phase), the infected carcasses assume the role of the main epidemiological reservoir of ASFV; in this circumstance, wild boar density becomes of ancillary importance in the cycle. ASF is eradicated when the last infectious wild boar and the last infectious carcass are removed from the infected area.
Key messages
- ASFV survives in the wild boar population inhabiting northern and eastern Europe without any help from domestic pigs or ticks.
- ASFV is highly resistant in any matrix, and low temperatures increase its survival time.
- The infection spreads through both direct and indirect contact. The carcasses of infected wild boar maintain the live virus for a long time, especially during winter, allowing for indirect transmission when in contact with susceptible wild boar.
- Due to the epidemiological role played by carcasses, the simple mechanistic reduction of the wild boar population size has an ancillary value if carcasses are not removed and safely disposed; infected carcass presence allows for the persistence of the virus, even if the infected wild boar population is managed at extremely low density.
- The imprecise estimates of the wild boar population size and density, together with a lack of knowledge of the main epidemiological parameters of the transmission cycle, prevent any estimate of a possible density threshold for infection fade-out, and the critical size of the wild boar community required to modulate disease dynamics.
- Any depopulation approach should consider that:
- The introduction phase can be avoided only by interventions and preventive measures implemented in the source population, never in the receiving population.
- A successful invasion can be prevented or minimized by managing a wild boar population at the lowest possible density, but only before introduction has taken place.
- During the epidemic phase, chances of eradicating the disease are low (if any), simply due to the high number of infectious wild boar present, whereas the risk to promote further geographical spread of the virus is high.
- During the endemic phase, there is a certain probability of eradicating the infection through the reduction of the host community as much as possible, together with carcass removal under strict biosecurity measures.
- Continuous passive surveillance is the main tool for understanding the evolution of the disease (phase identification, geographical spread etc.).
Reference
Guberti, V., Khomenko, S., Masiulis, M. & Kerba S. 2022. African swine fever in wild boar – Ecology and biosecurity. Second edition. FAO Animal Production and Health Manual No. 28. Rome, FAO, World Organisation for Animal Health and European Commission. https://doi.org/10.4060/cc0785













