



Evaluation of Premix Characteristics and Pelleting Stability for Aureomycin Granular and Terramycin Meal Premixes in a Large Midwestern US Feedmill
A study was conducted at a large midwestern US feedmill comparing the formulation characteristics of AUREOMYCIN® granular chlortetracycline (CTC) premix and Terramycin® oxytetracycline (OTC) meal premix, reports Zoetis.Premix formulation has a dramatic impact on premix handling and feed distribution characteristics, potential drug loss during the feed manufacturing and delivery process, and drug stability under adverse conditions such as pelleting or harsh environmental temperatures. Ultimately, premix formulation has a direct effect on the amount of drug presented to the pig’s digestive tract for disease treatment or control.
A study was conducted at a large midwestern US feedmill comparing the formulation characteristics of AUREOMYCIN® granular chlortetracycline (CTC) premix and Terramycin® oxytetracycline (OTC) meal premix.1
Experiment Design
AUREOMYCIN 90 granular Type A premix (90g CTC per lb of premix, Zoetis/Alpharma Animal Health) and Terramycin 100 meal type A premix (100g OTC per lb of premix, Phibro Animal Health) were purchased by the feed mill in June 2002. Both products were within their expiration dating. Representative samples of each premix were collected and tested for potency, particle size, and drug activity distribution.2
Five consecutive batches of swine grower/finisher feed containing either Terramycin or AUREOMYCIN were manufactured. Each premix was added to the feed at 400g per ton according to label directions to provide drug dosing to animals at approximately 10mg per lb body weight. A soybean meal/corn flush was used between the AUREOMYCIN and Terramycin medicated feeds to clean-out the system. Batches 2, 3 and 4 were designated as the test batches for each drug.
After mixing each test batch, multiple ‘grab’ samples were collected from the mash leg and a representative composite sample obtained for assay. These mash leg composite samples were designated the ‘pre-pelleting’ samples. All feeds were then pelleted, with an average pelleting temperature of approximately 195°F. Three representative samples of cooled pellets were collected for each medicated feed at approximately 20, 25, and 30 tons throughput. To minimize assay variation, all assays were performed using HPLC.
Results
Premix potency was slightly above label (104 per cent) for both premixes.
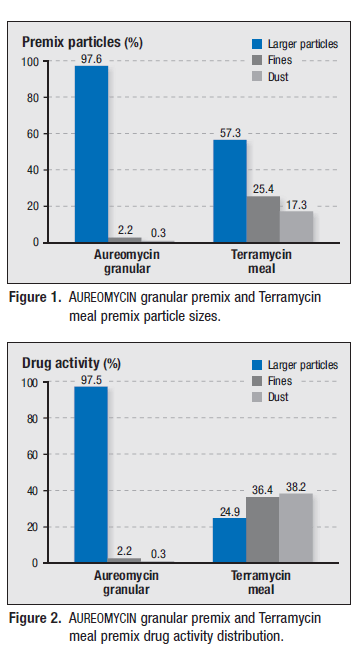
Results of the particle size and drug activity distribution evaluations are summarized in Figures 1 and 2.
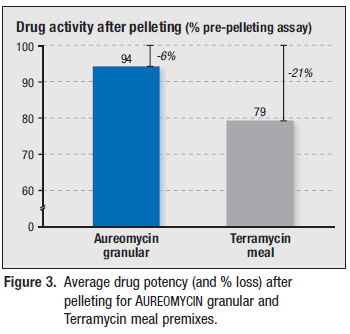
Pre-pelleting assays averaged 401g per ton and 378g per ton for AUREOMYCIN and Terramycin feeds, respectively. Post-pelleting assays for AUREOMYCIN averaged 376g per ton compared to only 298g per ton for Terramycin (Figure 3).
On average, AUREOMYCIN potency dropped by only six per cent during pelleting, compared to a 21 per cent loss of drug activity for Terramycin (Figure 3).
Discussion
Premix characteristics*
Premix potency results from this study differed from a previous study in which two lots each of Terramycin and AUREOMYCIN premixes were purchased from a local distributor and evaluated.3
In that study, the Terramycin premix potencies were 83 per cent and 89 per cent of label, while the AUREOMYCIN premix potencies were 99 per cent and 103 per cent.
In this study, AUREOMYCIN granular premix was comprised of predominantly larger particles that were shown to contain almost all of the drug activity while the Terramycin meal premix, in contrast, contained over 40 per cent fines and dust that held most (>75 per cent) of its drug activity.
Other studies have shown that fines and particularly dust may cling to the side of the mixer, be left behind in the auger system, end up in the dust collection system, or otherwise be lost, along with the drug activity, during feed manufacturing and delivery.4
Pelleting stability*
Antibiotics can be negatively impacted by adverse manufacturing conditions such as pelleting or extrusion. The pelleting process incorporates high temperatures, moisture (steam), and generates frictional heat as the feed is forced through the pellet dye.
The larger particles that predominate in AUREOMYCIN granular premix provide less surface area for exposure to these adverse conditions. The average surface area of the particles contained in the AUREOMYCIN granular premix used in this study was approximately 50 per cent less than for the particles found in the Terramycin meal premix.
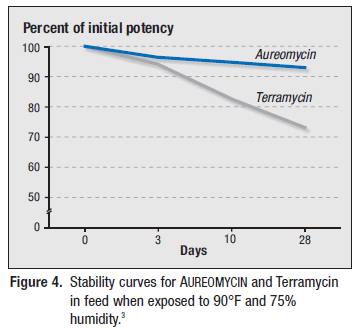
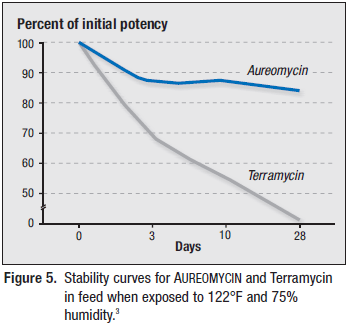
AUREOMYCIN has been shown to withstand adverse environmental conditions like high heat and humidity (i.e., conditions inside a feed bin during summer months) better than Terramycin (Figures 4 and 5).3
Conclusions
AUREOMYCIN granular premix consisted mostly of larger particles with less than three per cent fines and dust, and almost all of the CTC activity was found in these larger particles. In contrast, almost half of the Terramycin meal premix consisted of fines and dust that contained more than 75 per cent of its OTC drug activity. Studies have shown that the fines and dust are often lost during milling and delivery.
AUREOMYCIN granular premix withstood the rigors of pelleting better than the Terramycin meal premix, with AUREOMYCIN activity declining only six per cent compared to a 21 per cent loss of Terramycin potency.
AUREOMYCIN granular premix maintained high potency levels over time under adverse environmental conditions of high heat and humidity better than Terramycin meal premix.
AUREOMYCIN granular premix is formulated to potentially provide more drug than Terramycin meal premix to the pig’s digestive tract for superior disease treatment and control.
References
1. Wolff T. Evaluation of premix characteristics and pelleting stability for Aureomycin granular and Terramycin meal premixes in a large midwestern USA feedmill. Proc Am Assoc Swine Vet 2003; 185-190.
2. Kansas State University. Evaluating particle size. Cooperative Extension Service Bulletin MF-2051.
3. Data on file, Study Report TC4175, Zoetis Inc.
4. Laing N. Granulated ingredients: reducing drug potency loss. Feed Management 1994; December.
August 2013







