



Pigs with APP have less mortality, fewer lung lesions after treatment with Excede® for Swine compared to enrofloxacin
Pigs with swine respiratory disease (SRD) due to Actinobacillus pleuropneumoniae (APP) had significantly less mortality and fewer lung lesions when they were treated with Excede® for Swine (ceftiofur crystalline free acid) compared to pigs treated with enrofloxacin, a comparative challenge study demonstrated.1
Excede for Swine also provided better protection against secondary infections with Streptococcus suis compared to enrofloxacin.
For the study, 140 healthy commercial pigs were randomly assigned to one of seven treatment groups, each with 20 pigs (Table 1). Three of the treatment groups received one dose of Excede for Swine at either 3, 5 or 7 days before an intratracheal challenge with APP serotype 5, a strain that remains common in the US.2 Another three treatment groups received one dose of enrofloxacin at either 3, 5 or 7 days before the same challenge.
The seventh group received saline 3 days before challenge to provide a control. Excede for Swine and enrofloxacin were administered according to label directions.
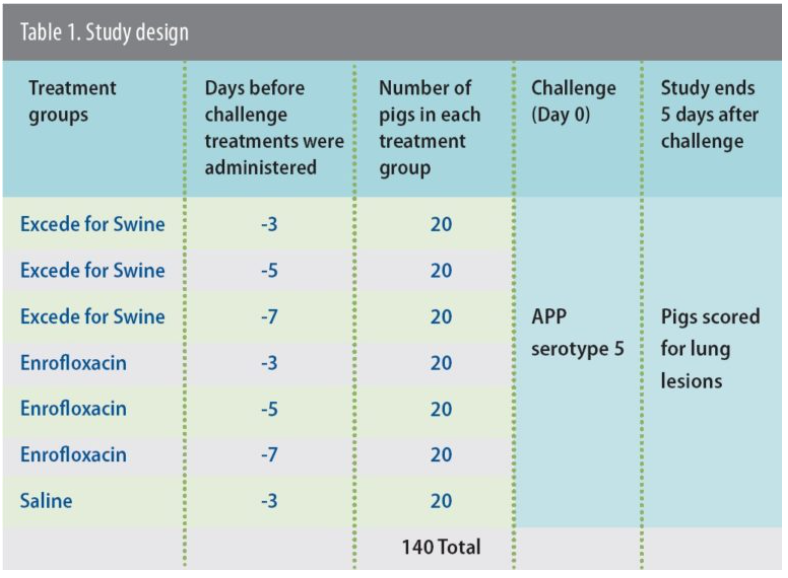
“Every group treated with Excede for Swine had less mortality compared to pigs treated with enrofloxacin (Figure 1) and the difference was significant (P ≤ 0.05),” reports Eva Jablonski, DVM, senior technical services veterinarian, Zoetis.
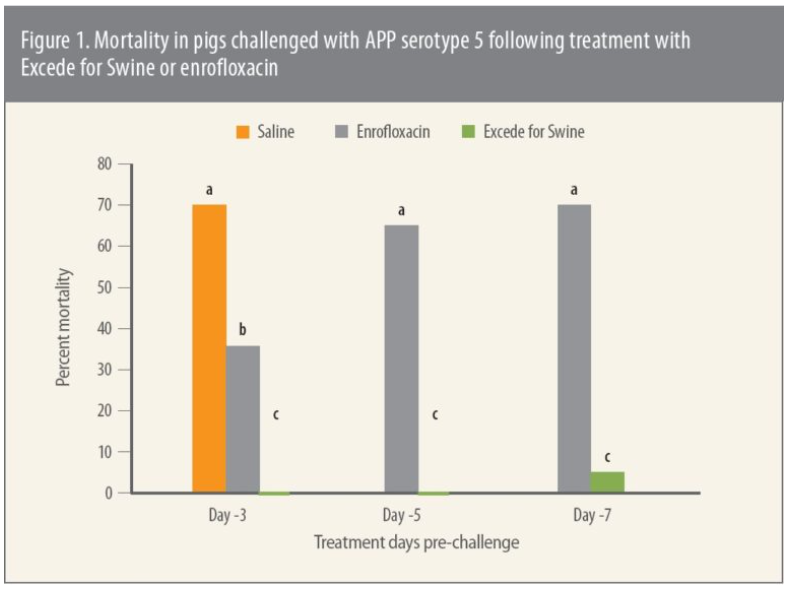
Lung lesion scores 5 days after challenge were based on the percent of gross lung involvement, she says. “The findings here too showed that every group treated with Excede for Swine had significantly (P ≤ 0.05) lower total lung lesion scores compared to enrofloxacin-treated pigs,” she says (Figure 2).3
“Excede for Swine was more effective for a longer period against APP compared to enrofloxacin. Even though both are broad-spectrum antibiotics, enrofloxacin provided only limited or no protection for 3 days. The ability of Excede for Swine to prevent losses for longer can amount to substantial savings for producers,” Jablonski says.
Protection against S. suis
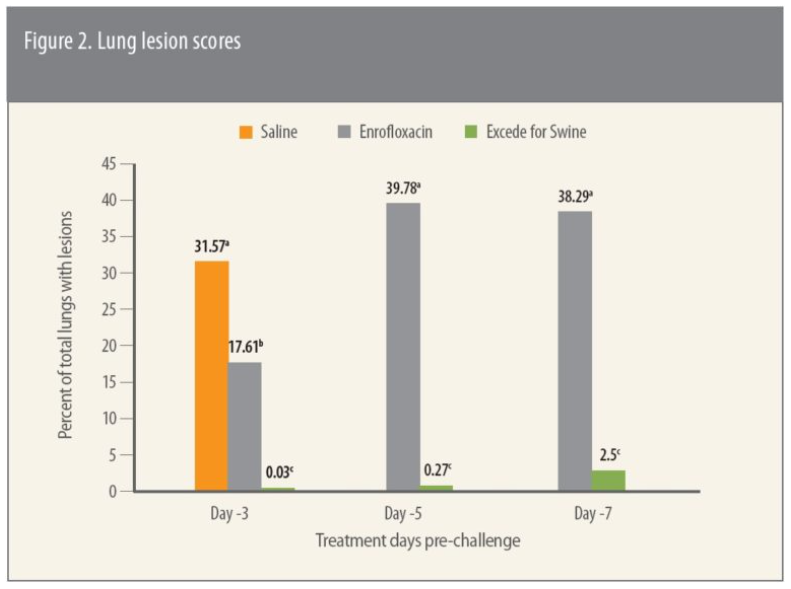
As part of the same study, investigators evaluated protection against bacterial growth with APP as well as S. suis, which often occurs as a secondary infection to APP. The assessment was based on 65 APP and 63 S. suis isolates isolated from challenged pigs.
Excede for Swine provided superior protection against both APP and S. suis compared to enrofloxacin in the study (Figure 3), she says.
Excede for Swine’s efficacy against S. suis as well as Haemophilus parasuis was also demonstrated in a recent study conducted in association with Iowa State University.4 Compared to an autogenous vaccination program and no treatment, pigs on a 4,000-head sow farm treated at weaning with Excede for Swine required significantly (P ≤ 0.05) fewer antibiotic treatments.
The authors of the study concluded that “By reducing the number of treatments postweaning, [Excede for Swine] improved pig health, decreased the labor associated with individual pig treatments and supported the responsible use of antibiotics on this farm.”
Excede for Swine is a third-generation cephalosporin approved for treatment or control of SRD associated with APP and S. suis as well as Pasteurella multocida and H. parasuis. Its unique, extended-release properties have also been demonstrated in a pharmacokinetic analysis, which showed that therapeutic plasma levels of ceftiofur and desfuroylceftiofurrelated metabolites are reached within just 1 hour after administration and are maintained for at least 7 days.5
The Zoetis antimicrobial susceptibility testing program monitors the susceptibility of APP, S. suis and P. multocida. All three pathogens have remained highly susceptible to ceftiofur since it was launched over 15 years ago,6 Jablonski says.
“By using an antibiotic that reduces the need for retreatments, producers will save on medication as well as labor costs. An effective antibiotic should also minimize performance losses, and all that adds up to better producer profits,” she says.
IMPORTANT SAFETY INFORMATION
People with known hypersensitivity to penicillin or cephalosporins should avoid exposure to EXCEDE FOR SWINE. Do not use in swine found to be hypersensitive to the product. Pre-slaughter withdrawal time is 14 days following the last dose. Click here to see full Prescribing Information.
| References | ||||
|---|---|---|---|---|
| 1 Data on file, Study Report No. ORPORK 2030, Zoetis LLC. | ||||
| 2 Actinobacillus pleuropneumoniae in swine. Iowa State University. | ||||
| 3 Data on file, Study Report No. ORPORK 2030, Zoetis LLC. | ||||
| 4 Finch M, et al. Evaluation of the efficacy of ceftiofur crystalline free acid versus autogenous vaccination for control of Streptococcus suis and Haemophilus parasuis in a commercial swine production system. Am Assoc Swine Vet. 2019. | ||||
| 5 Hibbard B, et al. Pharmacokinetics of ceftiofur crystalline free acid in swine. Proceedings of the 18th IPVS Congress, Hamburg, Germany 2004, Volume 2. | ||||
| 6 Sweeney M, et al. Antimicrobial susceptibility of Actinobacillus pleuropneumoniae, Pasteurella multocida, Streptococcus suis, and Bordetella bronchiseptica isolated from pigs in the United States and Canada, 2011 to 2015. J Swine Health Prod. 2017;25(3):106-120. | ||||







