



Rotaviral Diarrhoea in Pigs
The clinical signs, diagnosis and control of diarrhoea caused by rotavirus are outlined by Linda J. Saif and Anastasia N. Vlasova of Ohio State University in this factsheet from the Pork Information Gateway.Background
Group A rotaviruses were first detected in pigs suffering from diarrhoea in 1975. It is generally accepted that multiple rotavirus strains are present in most if not all conventional swine herds. Rotavirus infections are very prevalent and are commonly associated with diarrhoea in suckling and weaned pigs.
Early studies also demonstrated that porcine rotaviruses are physically and serologically similar to rotaviruses recovered from other host species including humans. Originally, only rotaviruses sharing a common group A antigen were identified in swine. In 1980, viruses that resembled rotaviruses in physical appearance, size and biochemical composition were detected using electron microscopy on faecal samples from pigs with diarrhoea. However, these rotaviruses were serologically different (i.e. they did not share similar group A rotavirus determinants) from the previously identified conventional group A rotaviruses and hence did not react in diagnostic tests commonly used to detect group A rotavirus.
These non-group A rotaviruses that have been referred to by a number of names including pararotaviruses, rotavirus-like viruses, antigenically distinct rotaviruses, and atypical or novel rotaviruses are now classified as groups B and C rotaviruses.
Within a rotavirus group (A,B,C,E), the group members share similar viral determinants or antigens and thus cross-react with one another in various serological or diagnostic tests. However, there is no crossreactivity or cross-protection among the different groups of rotavirus, so vaccines for group A rotavirus do not cross-protect against group C rotavirus, etc.
Antibodies against both group A and C rotaviruses are found in nearly 100 per cent of pigs as they reach market weight. Detection of group C rotavirus is much more common (up to 56 per cent) in nursing pigs (seven days of age) while group A rotavirus was detected more commonly (up to 51 per cent) in post-weaning pigs (21 to 35 days of age).
Groups B, C and E rotaviruses are also associated with diarrhoea in swine.
Serological surveys have indicated that antibodies to non-group A rotaviruses belonging to B, C and E are common in most swine populations. Some human group A, B and C rotavirus strains are of suspected animal origin (porcine, bovine, rodents).
The Cause
Rotaviruses are characterised by their wheel-like appearance when viewed using the electron microscope. Rotaviruses are resistant to low pH, lipid solvents, and many commonly used disinfectants enabling them to survive for long periods under normal environmental conditions.
Like influenza viruses, rotaviruses have dual serotype/genotype designations, identified as G (for surface glycoprotein VP7) and P (for protease sensitive hemagglutinin VP4) types based on virus neutralization or genotyping assays. This is important because rotaviruses with distinct G and P types generally induce low cross-protection, so vaccines need to contain the dominant G and P types associated with the disease in the field.
At least four distinct types of group A rotaviruses have been identified in swine in the US including common G4 and G5 (protoype Gottfried and the OSU strains, respectively) and emerging G9 and G11 genotypes. Multiple P genotypes also occur that vary with G type, pig age group and region.
Over the last decade, improved diagnostic tools have allowed identification of the widespread group B and C rotaviruses in pigs suggesting that the previous conclusions about their lower prevalence may have been overshadowed by the higher prevalence, pathogenicity and zoonotic properties of porcine group A rotaviruses.
At least three different genotypes of group C rotaviruses were identified in pigs in the US, with recent group C rotavirus strains genetically distinct from the historic Cowden strain. Although some studies reported higher prevalence of group A and C rotaviruses than group B rotaviruses in pigs, genetically diverse group B porcine rotaviruses were recently characterized and reported to be highly prevalent in the US.
Pigs infected with one group or genotype are still susceptible to infection with another group or genotype. Healthy carrier sows may be faecal shedders during the peri-parturient period exposing their offspring to infection. Up to 30 per cent of healthy sows excrete group A rotaviruses around the farrowing period.
Clinical Signs and Epidemiology
Nursing pigs
Rotaviral diarrhoea generally occurs in nursing pigs at one to three weeks of age and is a cause of the clinical syndrome referred to as 'milk scours', 'white scours' or 'three-week scours'.
The age of peak incidence varies for the different rotavirus groups and under different management conditions. Probable reasons for the peak occurrence of group A rotavirus infections at two to three weeks of age include the decline in milk antibody levels, coupled with the dilution of this antibody as a result of the pigs ingesting creep feed and water. High levels of passive rotavirus antibodies in the colostrum and milk from the dam may temporary protect pigs but for unknown reasons, group C rotavirus infections are dominant in pigs under one week of age.
Rotavirus diarrhoea is characterised by a white or yellow stool which, at the onset, is liquid; but after a few hours or a day in uncomplicated cases, it becomes creamy and then pasty before returning to normal. In pigs sacrificed for postmortem, undigested milk is often evident in the intestinal contents, and the stomach is often full and distended with milk curd. Diarrhoea may persist for only a few hours or for several days. Vomiting may or may not be detected, but it occurs much less frequently than it does in enteric coronavirus infections including transmissible gastroenteritis (TGE) and porcine epidemic diarrhoea (PED).
Under ideal conditions, pigs remain active and usually lose little weight. Present information suggests that rotaviral infections in many pigs result in either no clinical signs of disease or only a mild disease characterised by short-term diarrhoea.
However, the severity of the disease, and the death rate may be increased by simultaneous infections with Escherichia coli (colibacillosis), TGE virus or other causes (other enteric viruses such as PEDV and sapovirus, clostridia, coccidiosis), by inadequate intake of immune milk, or by stressors such as chilling.
The disease is more severe in young pigs. Diarrhoea is more profuse and more noticeable in pigs that ingest a large amount of milk.
In many respects, rotaviral diarrhoea is similar to enzootic TGE (persistence of TGE infection in a herd). Sows are usually not sick in either disease. Usually the duration of diarrhoea is longer, and dehydration and death losses are greater in enzootic TGE than in rotaviral diarrhoea.
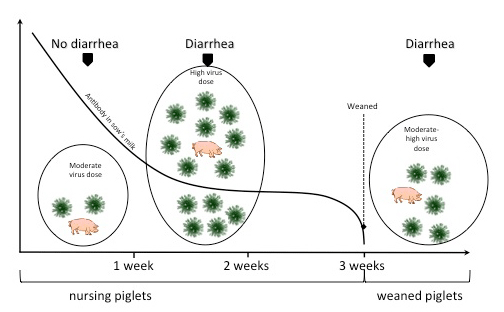
In continuous farrowing operations, rotaviral diarrhoea may initially be observed in two- to three-week-old pigs. As these pigs develop rotaviral diarrhoea, the environment becomes heavily contaminated with virus, which leads to exposure of younger pigs to high doses of virus often exceeding the protective capacity of the milk antibodies present in these pigs’ intestines (Figure 1). Subsequently diarrhoea may occur routinely in one- to two-week-old animals.
To break this cycle of infection, an “all in, all out” management system should be practised in farrowing and nursery units. Housing units should be designed with floors and all surfaces that can be thoroughly cleaned and disinfected between groups.
Weaning pigs
Pigs that have had rotavirus diarrhoea during the nursing period may have another episode about three to seven days after weaning. Whether these repeat episodes represent infections with different groups/serotypes of rotavirus is unknown.
Other researchers also have documented the importance of rotavirus as a cause of weanling diarrhoea. Such infections probably occur at weaning because of the loss of protective antibodies provided in the sow’s milk.
Two studies have shown that rotavirus infection shortly after weaning leads to intestinal damage which favours the colonisation of the gut with enteropathogenic E. coli. Results of both studies suggest that pigs infected with two agents develop a more severe diarrhoea than that produced by each agent alone.
Diet might also play an important role in weanling diarrhoea. A diet high in solids fed only three times daily produced a more severe and prolonged diarrhoea than either the same diet fed hourly or a similar diet containing one-third the amount of solids. Malabsorption resulting from rotavirus infection was most severe in pigs fed the diet high in solids, and this diet also favoured intestinal colonization by enteropathogenic E. coli.
Besides diet composition, other management variables that may influence the occurrence and severity of rotavirus and E. coli. weanling diarrhoea include:
- meeting the critical temperature needs of the pigs
- avoiding overloading the animal’s digestive system with too much food at one time
- isolating the nursery, disinfecting it between batches of pigs, and
- dividing pigs into smaller groups of similar ages.
The dynamics of virus-host interactions in rotavirus infection is illustrated in Figure 1. Three simple facts are useful in predicting whether rotavirus will be a problem:
- Rotavirus is widespread and highly stable in nature. Poor management practices such as continuous use of facilities without a cleanup, fumigation and resting time between groups of pigs increase the dose of microbes in the environment, including rotavirus.
- Most sows have protective antibodies in their milk and colostrum (gilts may have less and may require additional vaccinations). Rotavirus (like TGE and PED virus) grows in and destroys the cells of the gut. Therefore, to protect the pig’s gut cells from rotavirus, antibody must be present in the gut (antibody in the pig’s blood is not protective).
- The younger the pig, the more vulnerable it is to dehydration and energy and weight losses caused by rotavirus.
Keeping these facts in mind and referring to Figure 1, it is easy to see that pigs will have problems with rotavirus every time the dose of the virus exceeds the protective antibody level in the pig’s gut, this antibody being supplied by the sow’s milk. Therefore, the younger the pigs when weaned in a contaminated environment, the greater the chance that a severe outbreak of rotaviral diarrhoea will occur. In addition, the earlier weanings result in higher death losses if pigs develop rotavirus diarrhoea.
Even though pigs weaned at three weeks of age are somewhat resistant to rotaviral diarrhoea at this time, the abrupt removal of the pigs from the protective antibody in the sow’s milk leaves them vulnerable to the moderate dose of rotavirus that is in their environment. It is also true that if the dose of virus is high enough, pigs nursing immune sows will also experience rotaviral diarrhoea in the farrowing house.
How the Virus Causes Disease
Rotavirus, like TGE and PED virus, has a special affinity for cells which line the small intestine. These cells cover the millions of long fingerlike projections, called villi, which make up the inside lining of the small intestines. When these cells are infected and destroyed by rotavirus, the villi become short and blunt or shrink, and nutrients are incompletely digested and poorly absorbed.
In suckling pigs, much of the ingested milk will pass through the gut without being digested or absorbed. The passage of undigested food into the lower small intestine and large intestine has two effects:
- It provides a substrate for various bacteria lower down the gut leading to secondary disease, and
- It can have an osmotic effect in the large intestine preventing water re-absorption. This can result in diarrhoea, loss of water, electrolytes, body weight and sometimes death.
Villous atrophy occurs very rapidly, within 24 to 36 hours, after rotavirus infection of the intestinal cells, and coincides with the onset of diarrhoea. However, regeneration of the intestinal villi and recuperation of normal digestive capacities will take about seven to 10 days. This is then the most critical time to prevent malabsorption diarrhoea and secondary bacterial infections.
Diagnosis
Clinical and laboratory diagnosis of rotaviral diarrhoea requires also evaluation for the presence of E. coli, Isospora suis (coccidiosis), enzootic TGE, and other agents that can cause a similar diarrhoea syndrome.
E. coli diarrhoea commonly occurs in younger (one week old or less) pigs, or at four to 10 days post-weaning, whereas enzootic TGE, coccidiosis and rotavirus diarrhoea often occur in pigs after one week of age. However, since disease caused by rotavirus in younger pigs is usually more severe, producers might think the pigs have colibacillosis unless they submit pigs for a complete diagnosis.
Laboratory diagnosis requires the submission of faeces or intestinal sections collected early (24 hours or less) after the onset of diarrhoea. Laboratory methods that are helpful in making a diagnosis (when used in combination), include: histopathology, electron microscopy (EM), fluorescent antibody (FA), reverse-transcription polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA).
Faeces or intestinal contents can be examined for viral particles using electron microscopy. However, an electron microscope may not be available in all diagnostic laboratories, and this technique cannot discriminate between group A rotaviruses and the morphologically identical non-group A rotaviruses. The non-group A rotaviruses and other viruses can be differentiated from group A rotavirus using serologic tests such as immune EM, FA, and ELISA, all of which should employ highly specific antisera for each virus.
Currently, commercial FA and ELISA reagents are available only for detection of group A rotaviruses. For FA, one of the most commonly used tests, pigs in the early stages of diarrhoea must be sacrificed. Scrapings or sections are made from the lining of the small intestines and stained with antibodies to rotavirus conjugated to a fluorescent dye (FITC). Cells infected with rotavirus react with the FITC antibody and emit a bright apple-green fluorescence when excited by a certain wavelength of light.
In the ELISA, antibody to rotavirus is coated onto the bottom of a small plastic well or tube. The suspected faecal sample is added to the well. If rotavirus is in the sample, it is captured by the antibody on the plastic. Then another antibody to the rotavirus is added. This antibody has an enzyme conjugated to it. If rotavirus has been captured from the faeces, then the enzymeconjugated antibody will adhere to it. Finally, a substrate that produces a visual color change in the presence of the conjugated enzyme is added. If colour is produced, that enzyme-conjugated antibody is assumed to have bound to the captured rotavirus; hence, the sample is positive for rotavirus.
ELISA can be done on faeces or intestinal contents and has the advantages of high sensitivity, requirement for minimal amounts of sample, and rapid results (six to 24 hrs). RT-PCR tests can also be performed after rotaviral nucleic acid is extracted from a suspended faecal sample. If rotavirus nucleic acid is present in the sample (it can only be present if rotavirus is present), it will be recognised as rotavirus-specific DNA fragments that are amplified in a positive reaction when sufficient amounts of rotavirus nucleic acid are present in the sample. Amplified rotavirus nucleic acid can be visualized under UV light using a special fluorescent dye.
Blood samples aid little in serologic diagnosis, since most swine are positive for rotavirus antibodies.
Immunity
Because most if not all sows are positive for rotavirus antibodies, they will transfer a variable amount of passive immunity to their nursing pigs via colostrum and milk. Studies on immunity to TGE virus have shown that effective protection depends not on blood antibody levels, but on the almost continual presence of milk antibodies and other immune factors in the intestine of the pig, such as occurs following frequent nursing. This type of “lactogenic immunity” is also important in rotavirus infections for protection of susceptible intestinal cells.
Various factors which may interfere with this balance between passive immunity and rotavirus clinical infections include:
- failure of the pig to nurse at frequent intervals shortly after birth or failure of the sow to provide milk may lead to severe rotavirus diarrhoea in pigs under a week old;
- high doses of virus as a result of a heavily contaminated environment may exceed the level of protective antibodies in the milk, leading to rotavirus diarrhoea in nursing pigs;
- ingestion of creep feed and water by two- to three-week-old nursing pigs may dilute the level of protective antibodies leading to rotavirus diarrhoea; and
- weaning, which results in complete loss of protective milk antibodies, may cause severe diarrhoea and death losses to younger pigs which are weaned in a contaminated environment.
Parenteral (intramuscular or subcutaneous) rotavirus immunisation of rotavirus antibody positive sows shortly before or after farrowing can increase rotavirus antibody levels in colostrum and milk. T
he practical application of these immunisation methods might be to enhance passive lactogenic immunity, thereby delaying the onset of rotavirus diarrhoea in herds with a history of severe rotavirus diarrhoea and high mortality in pigs under two weeks of age.
Protection of weanling pigs against rotavirus diarrhoea requires active immunization, probably via the oral route, prior to weaning. Multiple serotypes of porcine rotavirus and interference by maternal antibodies make this type of potential vaccine a less feasible prospect at present.
Vaccines
Currently, only one manufacturer produces a federally licensed vaccine, available in different combinations, for porcine group A rotavirus. The most recent vaccine incorporates two serotypes of porcine group A rotavirus and is to be administered orally plus intramuscularly to pregnant swine or orally to nursing piglets.
In theory, administration of a rotavirus vaccine to pregnant swine should boost colostrum and milk antibodies providing increased lactogenic immunity to nursing pigs. However, there are no reported controlled studies on the efficiency of this vaccine for boosting rotavirus antibodies in colostrum and milk or for preventing rotavirus-associated diarrhoea in nursing pigs.
Current knowledge indicates that group A rotavirus vaccine failure can be due to co-circulation of multiple rotavirus serotypes other than the prototype vaccine strains or interference by maternal antibodies with active immunisation of pigs. Although an increasing problem in swine herds and especially in pigs under one week of age, there are no licensed vaccines for non-group A rotaviruses such as group C rotavirus.
Sows are often given rotavirus containing faecal material approximately two to five weeks before their expected farrowing date in an attempt to boost their immunity and enhance transfer of maternal immunity to the piglets. Efficacy of this feed-back approach has been variable and it may spread other enteric pathogens throughout the herd.
Prevention and Control
There is no specific treatment other than supportive therapy for rotaviruses.
Although most studies suggest that rotavirus infections cannot be prevented, their severity can probably be moderated by optimal management conditions. These include “all in/all out” systems in farrowing and nursery units. Careful and thorough cleaning and disinfection of the premises should be done routinely since high viral doses may lead to earlier onset of and possibly more severe infections in nursing pigs.
Disinfectants which are effective to various degrees against rotavirus include: 3.7 per cent formaldehyde, chloramine T (Multichlor®), 5 per cent Lysol, hexachlorophene (Septisol®), lime; and triclosan (Triclosan® hand soap).
It is likely that faecal material may further reduce the effectiveness of many of these rotavirus disinfectants, necessitating complete cleanliness to achieve maximal disinfection.
Attention should be given to providing adequate heat to suckling and weaned pigs since this affects their clinical response to rotaviruses and other enteric infections. Pigs with diarrhoea caused by rotaviruses or other infections that damage the villi do not absorb nutrients well and are more susceptible to chilling.
Although villous repair should occur within a few days, chilling and other stresses may delay this, and the pig may develop multiple nutritional deficiencies and become stunted or a chronic “poor-doer.” It is essential to ensure that neonatal pigs receive adequate colostrum and milk.
Control of weanling diarrhoea may depend on factors such as:
- feeding newly weaned pigs small quantities of feed at frequent intervals for the first few days post weaning
- dividing pigs into small groups of similar ages since mixing pigs of various ages at weaning may lead to stress and favor transmission of infection from older to younger pigs
- emptying and disinfecting the premises between groups
- weaning age: younger weaned pigs usually are more severely affected than older pigs
- meeting critical temperature needs of pigs; and
- ventilating for minimal levels of noxious gases (ammonia).
It is helpful to provide adequate water to maintain hydration in weaned pigs with diarrhoea.
Antibiotics or other drugs are not effective against rotaviral infections and would be of no value in treatment unless there is a concurrent bacterial infection, such as with pathogenic E. coli.
Summary
Ten points can be summarised from this fact sheet:
- Porcine group A rotavirus was first detected from diarrhoeic pigs in 1975.
- In 1980, non-group A rotaviruses were detected in swine and found identical in appearance but serologically distinct from conventional group A rotaviruses. The earliest detected rotaviruses are now classified as group A rotaviruses and the non-group A porcine rotaviruses are classified as groups B and C rotaviruses.
- Infection of swine with rotaviruses is very common and widespread. Probably all swine herds are infected.
- Rotavirus is frequently associated with a diarrhoea syndrome commonly referred to as white scours, milk scours, or three-week scours. diarrhoea is most frequently observed in one- to four-week-old suckling pigs or in pigs weaned around two to four weeks of age or earlier.
- Less is known about the prevalence or severity of infections with non-group A rotaviruses.
- The infection and diarrhoea caused by rotaviruses resembles that seen in coccidiosis and enzootic transmissible gastroenteritis but is less serious than the latter infection.
- Laboratory diagnosis of rotaviruses can be made by fluorescent antibody staining of mucosal scrapings from the small intestine or immune EM, ELISA or RT-PCR tests done on feces.
- Diagnosis of rotavirus solely by electron microscopy (EM) may not be accurate because of non-group A rotaviruses; diagnosis and differentiation of these viruses from group A rotaviruses requires use of specific antisera in immune EM or fluorescent antibody staining or RT-PCR tests using group or genotype specific primers.
- Death loss in suckling pigs is usually very low unless there are complications owing to concurrent infections or stress such as chilling. Reasons for the widespread presence and deaths associated with group C rotaviruses in pigs
- Present control measures must rely on good management such as ensuring that pigs get adequate colostrum and milk at an early age, providing good sanitation, and keeping pigs comfortable, especially warm and hydrated. Protection against pathogenic E. coli by effective immunization or other means may help reduce the severity of rotaviral diarrhoea in those herds having combined infections. Likewise simultaneous infections with group A and non-group A rotaviruses may increase the severity of the diarrhoea or death losses.
September 2013






