



Safety and Efficacy of a New Vaccine for Preventing Porcine Atrophic Rhinitis
The efficacy of Rhiniseng was demonstrated by means of a challenge in the period of maximum susceptibility of piglets to atrophic rhinitis, according to Jordi Montané, Daniel Torrents, J.A. Mesonero Escuredo, Ricard March and Marta Sitjà of Hipra.Introduction
Porcine atrophic rhinitis is an infectious disease, which, in the early
stages, is characterised by sneezing and nasal discharge that can vary
between serous and mucopurulent. Atrophy of the nasal turbinates and
nasal septal deviation can cause an upper brachygnathia of the snout.
The most severe form of the disease is caused by infection with toxigenic
strains of Pasteurella multocida either alone or in combination with
Bordetella bronchiseptica (progressive atrophic rhinitis). Infections in
which only B. bronchiseptica is involved cause a milder form of the
disease called non-progressive atrophic rhinitis, which is characterised
by a mild to moderate atrophy of the turbinates (de Jong, 1999).
Current vaccines against porcine atrophic rhinitis are mainly oil-based
emulsions or oil-based suspensions that cause obvious or marked
local reactions or temperature increases post-vaccination.
Rhiniseng®
(HIPRA) is a new vaccine formulated with an aqueous adjuvant,
Hipramune Gd, which minimises the occurrence of local reactions,
compared with oil emulsions and reduces temperature increases
compared to oil-based suspensions.
This study aims to demonstrate that the efficacy of the vaccine is not compromised by improving its
safety.
Objectives
The objective of this study was to evaluate the safety and efficacy of a new vaccine for preventing porcine atrophic rhinitis by performing a challenge. The main variable for assessing vaccine efficacy was the reduction of atrophic lesions of the turbinates and nasal septum deviation caused by Bordetella bronchiseptica and toxigenic Pasteurella multocida. To evaluate safety, the authors used rectal temperature increase, the occurrence of systemic and local reactions and alteration of reproductive parameters.
Material and Methods
Fifteen pregnant sows sero-negative to Bordetella bronchiseptica and
toxigenic Pasteurella multocida were selected for this study. They
were from a farm with no clinical signs of atrophic rhinitis. Sows were
vaccinated six weeks before the expected date of farrowing and revaccinated
three weeks later. The sows were divided into three treatment
groups based on number of parturitions.
The treatment that was given
to each treatment group was randomised. Five sows were vaccinated
with Rhiniseng (HIPRA), five sows were vaccinated with a commercial
vaccine with an oil-based adjuvant with vitamin E (α-tocopherol) and
the remaining five received phosphate buffer as a placebo treatment.
The rectal temperature of sows was recorded on the day before
vaccination, just before inoculation, at two, four and six hours after applying
the treatment and daily for the following three days. Local reactions
were assessed by thorough inspection and palpation of the entire side
of the neck in which the inoculation was administered with an additional
evaluation of reactions and effects on gestation.
The number of mummified piglets, the number of stillbirths, the litter
size and any alterations that could affect the progeny of each sow
were recorded at the time of farrowing.
Piglets born to sows were left with their mothers until 5 days of age.
On day 5, piglets were weaned and transferred to the biosafety
facilities where they were subjected to infection with toxigenic P.
multocida (Pm Group) or a combined infection with B. bronchiseptica
and toxigenic P. multocida (Bb + Pm group).
To form these challenge
groups, six piglets per sow were randomly selected from a total of four
sows from each treatment group. The six piglets from each litter were
divided into two separate isolation rooms (three pigs per room). Thus, three of
the piglets from each litter were challenged with Bordetella bronchiseptica
at seven days of age and with Pasteurella multocida at 10 days of
age (Bb + Pm group), while the other three piglets from the same litter
were infected with Pasteurella multocida at 10 days of age (Pm
group).
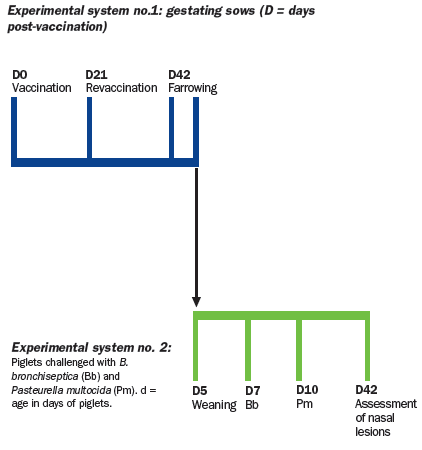
To form the non-challenged control group (sentinel group), two piglets
were randomly selected (from two different litters) from each of the groups
of sows to form a group of six animals.
Two days before the challenge, 1ml of acetic acid solution (10g per litre) was
instilled intranasally (0.5 ml per nasal cavity) to cause irritation of the nasal
mucosa prior to inoculation with pathogens of atrophic rhinitis. This
treatment is especially necessary when the challenge is performed with only
toxigenic P. multocida, as it is unable to colonise the nasal mucosa without
previous irritation caused by environmental factors or by a prior infection with
B. bronchiseptica.
The B. bronchiseptica inoculum for the challenge was prepared with strain
4609, producing a dermonecrotic toxin that is pathogenic in pigs
(Ackermann et al. 1991; Register and Ackermann, 1997). The inoculum
contained 2×108 colony forming units per ml (CFU / ml). Inocula of P.
multocida were prepared with strain NCTC 12178, a highly toxigenic capsular
type D strain (Foged et al., 1988) isolated by J.P. Nielsen (1978). The
piglets included in the Bb + Pm group received 1ml (0.5ml per nasal cavity)
of an inoculum with 6.9×108 CFU per ml, while piglets included in the Pm group
received 2ml (1ml per nasal cavity) of an inoculum with 4.4×109 CFU per ml.
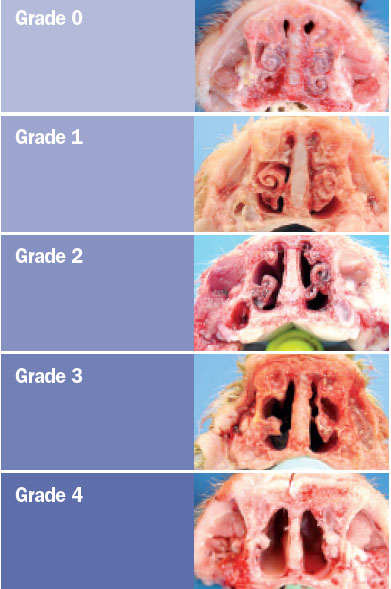
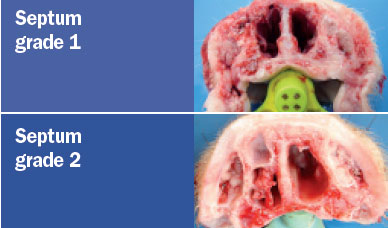
The animals were sacrificed at 42 days of age and the snout was sectioned
between the first and second premolar area to assess the degree of atrophy of
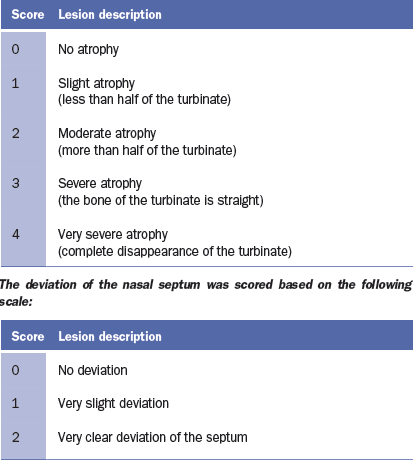
the turbinates and nasal septal deviation. The scoring system for assessing the
degree of atrophy in each of the ventral and dorsal nasal turbinates was as
follows (Magyar et al., 2002; Monograph 1361 of the European Pharmacopoeia).
The nasal lesion score (NLS) was obtained using the total sum of the
degree of the four turbinates (maximum score 16) and the degree of nasal
deviation (maximum score of 2), for a maximum total score of 18.
The staff responsible for scoring the clinical and reproductive variables and
those responsible for the laboratory analysis conducted the study 'blind'.
Statistical Analysis
To analyse rectal temperatures, the authors used a mixed model of variance. The reproductive data were analysed using a One-Way ANOVA and Kruskal- Wallis test. Nasal lesion score was analysed using an analysis of variance (ANOVA) with a post hoc Dunnett test for comparisons of pairs. These analyses were performed using the SPSS 11.5 statistical program. The level of significance accepted in all cases was p<0.05.
Results
Rhiniseng vaccine caused a significantly lower temperature increase than
the commercial oil-based vaccine, especially in the period between four and six
hours post-vaccination (Figure 1). When re-vaccinating, the only vaccine
that induced an increase in rectal temperature was the oil-based vaccine.
Vaccination did not give rise to local reactions at the injection site. On
palpation, a slight protrusion on the skin corresponding to the entry point of
the needle was observed in some cases. This finding also appeared in
sows in the control group that had received PBS in a proportion similar to
that recorded in the vaccinated sows, so it was considered a reaction to
physical damage caused by the needle rather than a vaccine reaction. After
revaccination, no local reactions were observed by inspection. On palpation,
a mild transient swelling that grew to a maximum of 3cm in diameter was
detected in pigs vaccinated with Rhiniseng.
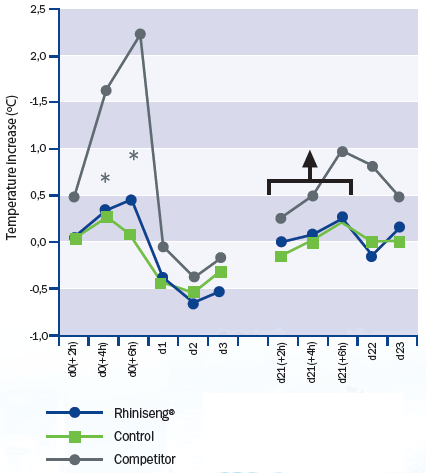
* There are statistically significant differences (mixed model analysis of the variance with 'unstructured' type covariance matrix; p<0.05) at 4 and 6 hours post-vaccination between the vaccine Rhiniseng and the competitor vaccine.
There is a significant increase in rectal temperature (mixed model analysis of variance with 'unstructured' type covariance matrix; p<0.05) in the period between revaccination and 6 hours post-revaccination only in animals vaccinated with the competitor's vaccine.
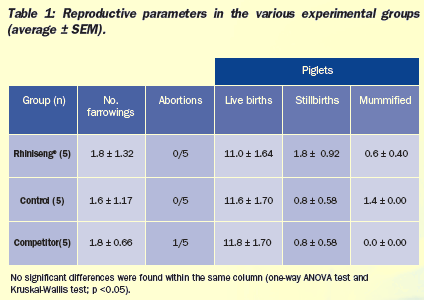
No significant differences were found in reproductive parameters
(Table 1). One of the five sows vaccinated with the competitor's
vaccine had an abortion. This was the sow that showed the highest
temperature increase both after vaccination (2.64°C) and after
revaccination (1.89°C).
Nasal lesion score, the main parameter for efficacy of vaccines
against porcine atrophic rhinitis, was significantly lower in piglets from
the vaccinated sows than in those from the control sows (Figure 2).
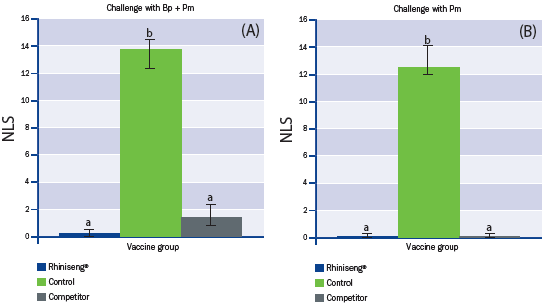
a,b Values with different superscripts are statistically different between experimental groups (One-Way ANOVA, p<0.05).
Conclusion
The efficacy of Rhiniseng was demonstrated by means of a challenge
in the period of maximum susceptibility of piglets to atrophic
rhinitis.
The strain of B. bronchiseptica used was a phase I strain,
i.e. in a virulent phase and producing a dermonecrotic dermonecrotic
toxin (Register and Ackermann, 1997) and capable of producing
lesions of non-progressive atrophic rhinitis. It has recently been
shown that expression of the dermonecrotic toxin by B. bronchiseptica
is not a necessary factor in predisposing to infection against
toxigenic P. multocida (Brockmeier and Register, 2007); therefore,
the strain used in this challenge was more virulent than necessary
to reproduce an image of progressive atrophic rhinitis. Nevertheless,
Rhiniseng protected pigs subjected to combined infection with
toxigenic strains of B. bronchiseptica and P. multocida.
In addition to providing adequate protection against challenges,
Rhiniseng caused a significantly lower rectal temperature increase
than that caused by the competitors' vaccine and, unlike the competitors'
vaccine, it did not cause abortion.
Bibliography
Ackermann M.R., Rimler R.B. and Thurston J.R. 1991 Experimental model
of atrophic rhinitis in gnotobiotic pigs. Infection and Immunity, 59:3626-3629.
Brockmeier S.L. and Register K.B. 2007. Expression of dermonecrotic toxin
is not necessary for predisposing to infection with toxigenic
Pasteurella multocida. Veterinary Microbiology, 125:284-289.
De Jong M.F. 1999. Progressive and Nonprogressive Atrophic Rhinitis.
In: Diseases of Swine. Ed. Straw B.E., D’Allaire S., Mengeling W.L. and Taylor D.J. Iowa University Press, Ames (IA). 355-384.
Foged N.T., Nielsen J.P. and Pedersen K.B. 1988. Differentiation of toxigenic
from nontoxigenic isolates of Pasteurella multocida by enzymelinked
immunosorbent assay. Journal of Clinical Microbiology, 26:1419-20.
Magyar, T., King V.L. and Kóvacs F. 2002 Evaluation of vaccines for
atrophic rhinitis - a comparison of three challenge modes. Vaccine, 20:1797-1802.
European Pharmacopeia Monograph 1361. Inactivated vaccines
against progressive atrophic rhinitis.
Register K.B. and Ackermann M.R. 1997. A highly adherent phenotype
associated with virulent Bvg+-phase swine isolates of Bordetella
bronchiseptica grown under modulating conditions. Infection
and Immunity, 65:5295-5300.
Further ReadingFind out more information on atrophic rhinitis by clicking here.Go to our previous article in this series by clicking here. |
December 2012







