



Evaluation of Late-Gestation Gilts and Sows Following Accidental Exposure to Fostera PRRS Virus
Two Zoetis studies reported here confirm that accidental exposure to the Fostera PRRS vaccine virus at 90 days of gestation does not compromise the health or farrowing performance of naive sows/gilts.In recent years, management practices aimed at controlling porcine reproductive and respiratory syndrome virus (PRRSV) have become mandatory to help ensure profit potential for pork producers. The cumulative impact of PRRSV on the US pork industry is estimated to exceed $1 billion per year due to production-related losses and other disease-control expenses.1 Thus, vaccination and other control measures are required to help growing pigs defend themselves against PRRSV and help producers avoid economic calamities.
Consistent use of a modified-live PRRSV vaccine in young growing pigs offers the most cost-effective tactic for PRRS control. However, modified-live PRRS viruses have the ability to replicate in vaccinated animals, with subsequent shedding of the vaccine virus into the animal’s environment. As a result, PRRSV-naive/negative swine can be exposed to vaccine viruses through biosecurity breaches, contact with vaccinated swine, or unintentional vaccination.
This scenario is particularly important for pregnant gilts and sows. Exposure of pregnant swine to field strains of PRRS can be devastating, depending on the virulence of the strain. Gestating dams may abort their litters, or the viability of live-born piglets may be jeopardized. The question facing veterinarians is whether the virus used in a modified-live vaccine will pose similar threats to farrowing performance when PRRSV-naive gilts and sows encounter the vaccine virus. Therefore, when selecting a PRRSV vaccine, it is vital to understand how the vaccine virus may affect pregnancy and/or piglet viability if naive, gestating animals are acciden tally exposed to the virus.
Fostera™ PRRS
Fostera PRRS, from Zoetis, is the first and only PRRSV vaccine that helps prevent respiratory disease associated with PRRSV with a duration of immunity of at least 24 weeks. Fostera PRRS helps optimize performance by minimizing the adverse affects of a subsequent PRRSV challenge, thereby allowing growing pigs to maximize their post-challenge weight gain.2 Fostera PRRS is the outcome of breakthrough research by Zoetis scientists that discovered a key cellular receptor protein for the PRRS virus, thus allowing for creation of unique cell lines for the growth and attenuation of PRRS viruses. Fostera PRRS offers a single-dose, easy-to-use formulation containing a modified-live PRRSV created from a US field strain that was attenuated through 52 passages on an innovative non-simian cell line, with no reversion to virulence.3
Fostera PRRS is approved for the vaccination of healthy, susceptible swine 3 weeks of age or older in PRRSV-positive herds or seronegative pigs deemed at risk for exposure to PRRSV as an aid in preventing respiratory disease associated with PRRSV. A 24-week duration of immunity has been demonstrated. Fostera PRRS is administered as a single 2-mL intramuscular (IM) dose, after aseptic rehydration of the freeze-dried vaccine with sterile diluent provided.
Vaccine virus may be shed and transmitted to other populations of swine in direct or indirect contact with vaccinated swine. The duration of potential vaccine virus transmission may vary. Non-vaccinated pigs in contact with Fostera PRRS-vaccinated pigs may seroconvert to vaccine virus. Use of the vaccine in herds intended to remain PRRS virus negative is contraindicated. Introduction of vaccinated pigs into herds intended to remain PRRS virus seronegative is contraindicated.
Two studies were conducted to evaluate the impact of Fostera PRRS vaccine virus exposure in pregnant swine, simulating conditions whereby breeding animals might become accidentally exposed to the vaccine virus. These studies posed a substantial test of the virus-attenuation efforts used in the creation of Fostera PRRS by not simply relying on animal-to-animal contact for virus transmission, but by injecting the vaccine into naive gilts and sows at 90 days of gestation. The aggressive degree of virus exposure used in these studies comprised a ‘worst-case’ scenario aimed at addressing customer concerns as well as responding to key opinion leaders, and outcomes are presented consistent with Zoetis goals to generate sound scientific data in a transparent manner. Both studies were conducted in accordance with the Zoetis Institutional Animal Care and Use Committee.
Experiment Design - Study A
The first exposure study involved 76 healthy pregnant gilts at approximately 90 days of gestation that were purchased from a SPF herd in Minnesota.4 All animals were confirmed free of PRRSV and PCV2 by PCR. Animals were Mycoplasma hyopneu moniae ELISA positive for antibodies. They were housed in a commercial farrowing unit with separate air spaces for each experimental group. Gilts were randomly allocated to 1 block of 3 animals each, and within blocks each animal was then randomly assigned to either control or exposed groups in a 1:2 ratio, respectively (randomized complete block design). One day later, animals in each treatment group received the following injections (study day 0):
- Fostera PRRS: 2 mL IM (n=51);
- Control: 2 mL vaccine diluent IM (n=25).
Data collected during the study included gilt morbidity and mortality, PRRSV serology, and litter productivity parameters (abortions, mummified/still-born piglets, live births, birth weights, pre-weaning mortality, weaning weights, birth-to-weaning daily gains). Serum samples collected from gilts (days -7, 0, 7, 48) and liveborn piglets were evaluated for anti-PRRSV antibody using a commercial ELISA test. Piglets that died were necropsied and selected tissue samples (lung, tonsil) were tested by PCR for detection of PRRSV. Remaining piglets were weaned at 21 days of age.
Collected data were statistically analyzed as least squares (LS) means by appropriate methods using each pig as an experimental unit, with statistical significance recognized at P≤0.05.
Results - Study A
Serology results summarized in Figure 1 show that gilts in the Fostera PRRS group generated higher (P≤0.0075) PRRSV antibody titers on days 7 and 48 compared to control animals. These data indicate that administration of Fostera PRRS was successful in challenging gilts with the vaccine virus for the purpose of evaluating possible accidental exposure responses. At no time during the study did the control animals ever become positive to PRRS (PCR or ELISA).
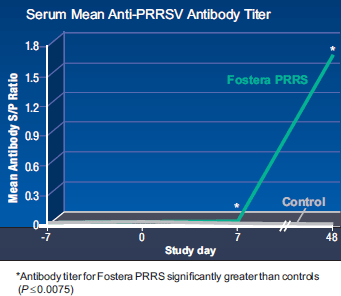
None of the gilts exposed to Fostera PRRS demonstrated any clinical signs of systemic disease associated with PRRSV, anorexia, or lethargy, nor did any control animals. No gilts in either group died.
Results of various farrowing parameters are summarized in Table 1. Most notably, no abortions occurred in any of the 51 gilts that received Fostera PRRS. In addition, no differences (P>0.56) were observed between treatment groups in the average number of pigs born/litter or pigs born live/litter. Similar rates of mummies occurred in each group, but a higher rate of still births was noted in the Fostera PRRS group. These outcomes were observed even though PRRSV viremia was detected at birth in 53 per cent of piglets born to gilts in the Fostera PRRS group (evidence of transplacental virus transmission). A higher rate of pre-weaning mortality was observed in the Fostera PRRS group.
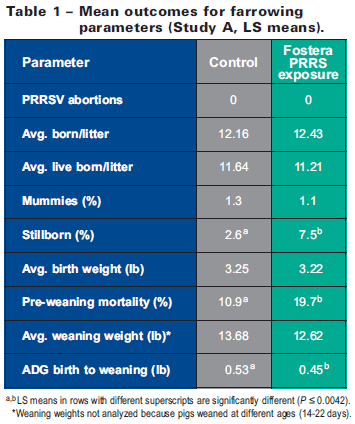
Though many piglets born to virus-exposed gilts were viremic at birth, they appeared clinically unaffected. Dyspnea was noted in five per cent of animals at 7 to 10 days of age, and 3 per cent were thin, gaunt, and rough-haired. Weaning weights were lower in exposed piglets, and average daily gain (ADG) during the birth-to-weaning period was reduced (P≤0.0001) compared to piglets born to control gilts (Table 1). These outcomes were not surprising given the fact that a full dose of vaccine virus was administered to 3rd-trimester, PRRSV-naïve gilts (worst-case scenario; Fostera PRRS is not licensed for use in PRRSV-naïve herds).
In summary, this trial demonstrated that inadvertent exposure of Fostera PRRS to naive gilts at 90 days of gestation posed no threat to farrowing success, with the most notable obser - vation being the absence of abortions and clinical disease that often result from PRRSV field infections.
Experiment Design - Study B
The second virus-exposure study5 followed a protocol similar to the first trial. The study was conducted at a major university and involved 15 healthy pregnant sows of mixed parity (2-10) that were confirmed free of PRRSV, PCV2, SIV, and M. hyopneumoniae. At 90 days of gestation, sows were randomly allocated to either control or exposed groups. On day 0 of the study, animals in each treatment group received the following injections:
- Fostera PRRS: 2 mL IM (n=10);
- Control: 2 mL vaccine diluent IM (n=5).
Sows were monitored for signs of clinical disease or lethargy throughout the 46-day trial. Rectal temperature and clinical signs of anorexia were recorded daily for one week and every other day for an additional week. Performance data were collected during gestation and at farrowing (abortions, mummified/still-born piglets, live births, birth weights, non-viable piglets, weaning weights, birth-to-weaning daily gains). Serum samples collected from sows at multiple time-points and from piglets at birth were analyzed for anti-PRRSV antibody (commercial ELISA) and viremia determination (PRRSV quantitative PCR). Piglets were weaned at 21 days of age. Sows were euthanized at day 46, with lung and tonsil tissues analyzed for the presence of PRRSV by PCR.
Collected data were statistically analyzed by appropriate methods using each pig as an experimental unit, with statistical significance recognized at P≤0.05.
Results - Study B
Serology results summarized in Figure 2 reveal that sows in the Fostera PRRS group generated PRRSV antibody titers from day 10 onward, compared to no antibody detection in control animals. These data indicate that Fostera PRRS administration was successful in challenging sows with the vaccine virus so performance parameters could be evaluated. Additional data further confirmed that sows in the Fostera PRRS group were indeed exposed to the vaccine virus:
- PRRSV viremia was detected by PCR in serum of all sows at day 3;
- PRRSV was found in tonsil and lung tissues at day 46 in 100 per cent and 40 per cent of sows, respectively.
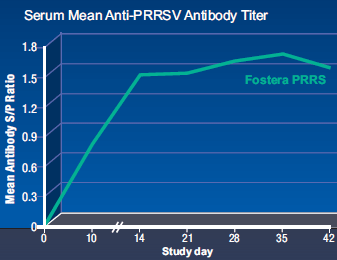
As observed in the first study, no clinical signs of systemic disease associated with PRRSV, anorexia, or lethargy were demonstrated by sows administered Fostera PRRS, and no animals died. One control sow aborted 9 piglets on day 7 (98 days of gestation), but gross lesions were not identified and no PRRSV was detected in lung or tonsil tissues at necropsy, so PRRSV was not diagnosed (sow removed from study). The remaining four control animals remained negative for PRRS virus and antibody during the trial and did not demonstrate clinical signs of systemic disease, anorexia, or lethargy. Rectal temperatures never exceeded 102.1°F in either treatment group between study days 0 and 13, though significant differences (P≤0.05) between groups were noted on day 0 (Fostera PRRS group higher) and day 13 (control group higher).
Results of various farrowing parameters (Table 2) reveal that no abortions occurred in any of the sows administered Fostera PRRS. In addition, no differences (P>0.05) were observed between treatment groups for average gestation length, number of pigs born/litter, pigs born live/ litter, birth weights, or rates of mummies, still borns, or non-viable piglets. These outcomes were observed even though PRRSV viremia was detected at birth in 84 per cent of piglets born to sows in the Fostera PRRS group (evidence of trans placental virus transmission).
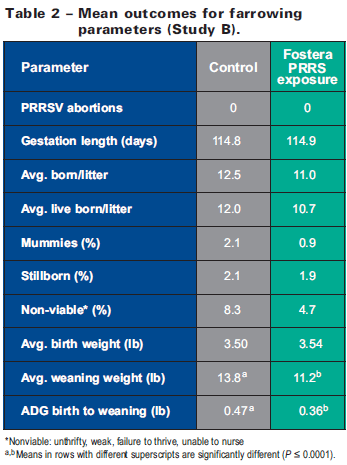
As in the first study, viremic piglets born to sows exposed to the vaccine virus appeared clinically unaffected. Some dyspnea was noted in two of 10 litters at seven to 10 days of age and piglets were thin, gaunt, and rough-haired; diarrhea affected 4 litters. Weaning weights were lower in exposed piglets, and ADG during the birth-to-weaning period was reduced (P≤0.0001) compared to piglets born to control sows (Table 2). Again, these outcomes were not surprising given the worst-case scenario of administering a full dose of vaccine virus into 3rd-trimester, PRRSV-naive sows.
This trial further demonstrated that exposure of naive sows at 90 days of gestation to Fostera PRRS vaccine virus did not cause reproductive failures or result in differences in farrowing performance compared to non-exposed controls.
Conclusions
Results of two studies confirm that accidental exposure to the Fostera PRRS vaccine virus at 90 days of gestation does not compromise the health or farrowing performance of naive sows/gilts. After administration of Fostera PRRS, no sows or gilts aborted their litters and no substantive differences in farrowing parameters were observed compared to animals gestating in the absence of virus exposure. Performance of piglets was as expected given the PRRS exposure under which they were reared.
Veterinarians and their clients can use Fostera PRRS in production herds with the confidence that the vaccine virus does not pose a hazard for the health or farrowing performance of PRRSV-naive gilts and sows following accidental exposure.
References
1. Holtkamp D, Kliebenstein J, Zimmerman J, Neumann E, Rotto H, Yoder T, Wang C, Yeske P, Mowrer C, Haley C. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on US pork producer. National Pork Board Research Report 2011; NPB #10-158.
2. Data on file, Study Report No. 3127R-60-10-890, Zoetis Inc.
3. Data on file, Study Report No. 3425R-60-09-790, Zoetis Inc.
4. Data on file, Study Report No. 12PORKORBI002, Zoetis Inc.
5. Data on file, Study Report No. 12ORBIOPORK04, Zoetis Inc.
August 2013







