



Is this clostridial disease the cause of sudden deaths in your herd?
Neglected but always present, Clostridia are gram-positive, sporulating, oxygen-tolerant to strictly anaerobic rods, many of which cause disease in pigs.by J. Glen Songer, PhD, Fellow, American Academy of Microbiology. Diplomat, American College of Veterinary Microbiology. Professor Emeritus, Arizona University.
1.What is Clostridium novyi?
1.1. Subtypes and toxins
Clostridia are gram-positive, sporulating, oxygen-tolerant-to-strictly anaerobic rods, many of which cause disease in pigs, other domestic animals, and humans (Figure 1). They can come from exogenous (most commonly the soil or animal faeces) or endogenous sources (often the intestinal tract of the affected individual) (Songer, 1996).
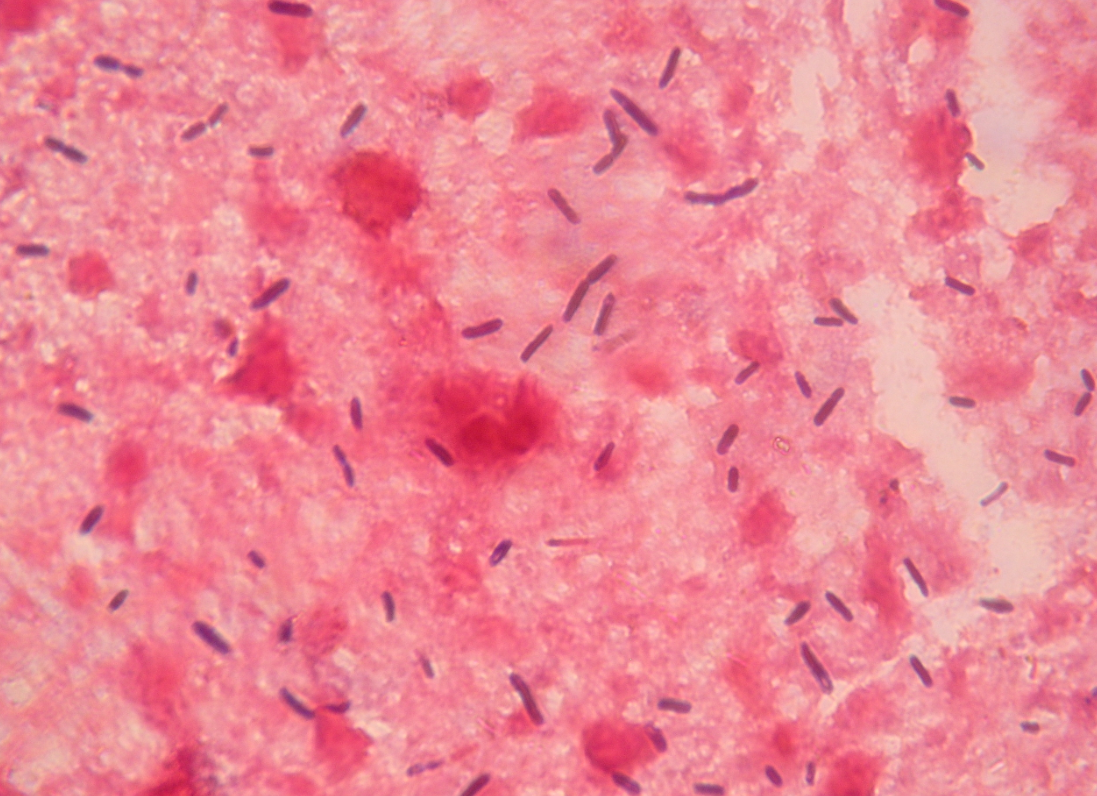
Figure 1. Smears from the liver of a sow that died of Clostridium novyi infection, showing large gram-positive rods with oval to cylindrical subterminal spores (magnification ×1000). Photo courtesy of Dr. Alfredo García Sánchez, Centro de Investigaciones Científicas y Tecnológicas de Extremadura (CICYTEX).
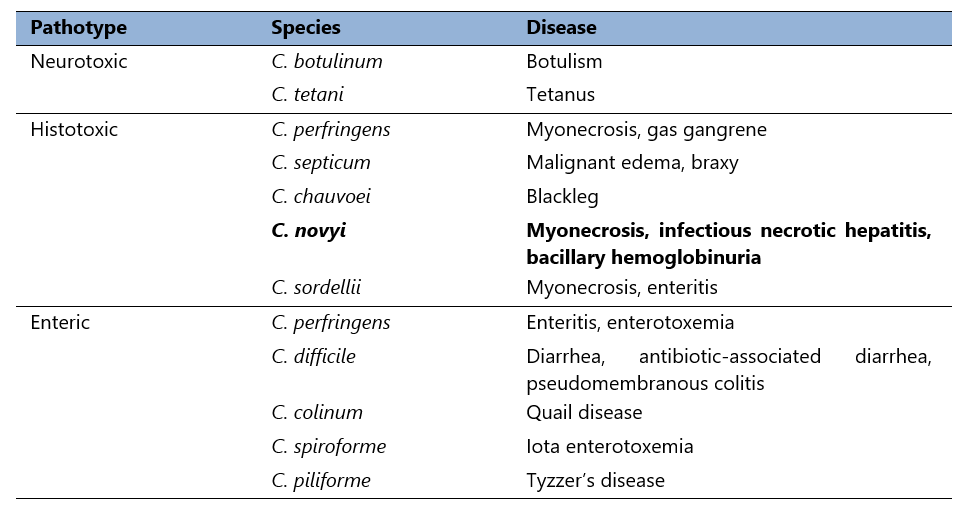
Pathogenesis of most clostridial infections is based upon production of toxins, and can be categorised as neurotoxic, histotoxic, and enteric as seen in Table 1 (Hatheway, 1990).
Table 1. Animal pathogenic clostridia.
Clostridium novyi was first isolated in the late 19th century (Novy, 1894). As noted (Table 2), it occurs as multiple types, differentiated by selective toxin production. Differential production of alpha and beta toxins determines the toxin phenotype. Clostridium novyi type C is nontoxigenic (and therefore avirulent), but types A, B, and D (C. haemolyticum), cause disease in humans and domestic animals.
Table 2. C. novyi selective toxin production.
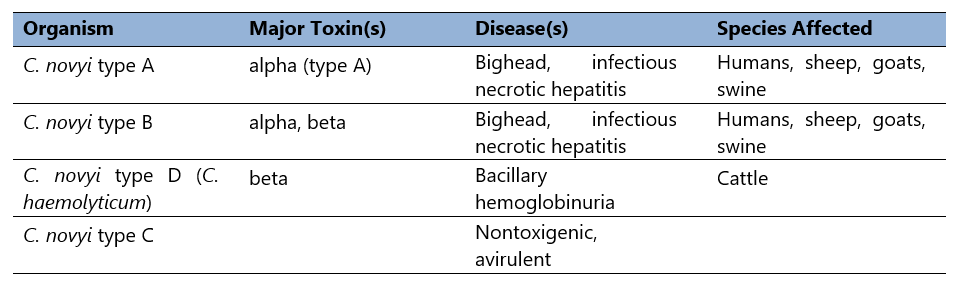
Thus, Clostridium novyi type A produces the organism’s alpha toxin. Type B, the primary cause of disease in sheep and swine, produces alpha toxin, but also makes a moderate amount of C. novyi beta toxin (Schranner et al, 1992). Finally, type D produces no alpha toxin, but secretes much more beta toxin than type B strains (Table 2).
It seems that C. novyi types B, C, and D are one independent species arising from the same phylogenetic background (Sasaki et al, 2002; Sasaki et al, 2001; Chen et al, 2011).
2. The need to be aware of porcine infectious necrotic hepatitis
There is nothing so disconcerting to a farmer or veterinarian on a pig farm as the sudden death of breeding sows. On the one hand, they are animals in which a large economical amount has been invested and on the other, a high mortality rate increases the renewal rate and therefore the percentage of gilts, with a consequent decrease in the general productivity of the farm (García, 2013).
2.1. Pathogenesis of C. novyi infections and toxin action
The local lesion, whether in humans or domestic animals, is a zone of necrosis. Alpha-toxigenic C. novyi strains cause disease that presents with an archipelago of signs and symptoms, including refractory hypotension, leukocytosis, and various effusions (Ryan et al, 2001).
All of the large clostridial cytotoxins consist of single peptide chains averaging about 250,000 MW. Its action upon injection is characterised by fluid loss into the interstitial space. Specifically, clostridial toxins glucosylate GTPases that are involved in actin cytoskeletal function impairing proper cell:cell contact (Selzer et al, 1996; Popoff and Bouvet, 2009), and leading to loss of integrity of vascular endothelium (Pires et al, 2012).
2.2. Epidemiology and transmission
It may be safe to say that, in the vast majority of cases, it is a peripartum disease. Rather, it is more probable that some physiologic event in proximity to parturition in the body of the sow is the inciting factor for disease.
Piglets could, conceivably, be colonised in the farrowing crate or stall during the first few hours after farrowing. In fact, it seems likely that exposure to a shedding sow early in life or to growing pigs at any stage is the most likely route to colonisation.
The majority of the sows experiencing infectious necrotic hepatitis are multiparous. Sows in good body condition are at greatest risk, and disease occurs most often in the summer. Most cases occur in lactating sows and losses can easily exceed 15–30% per year.
Colonisation without excretion (or simple shedding) seems a completely unreasonable expectation, so a colonised animal should be capable of spreading the organism to other pigs.
2.3. Clinical signs
The course of the disease is generally peracute, with hardly any clinical signs, which makes identification of sick animals difficult. The animals generally appear to be in good physical condition and then die suddenly, with unusual and rapid postmortem decomposition being characteristic (García, 2013).
Pathological changes include gross distention of the carcass, purple discoloration of the skin, generalised edema and subcutaneous infiltration with bubbles and foul smelling bloody fluid in pericardial, pleural, and abdominal cavities (Figure 2, A and B). All organs are typically softened, spongy, gas-filled, and severely necrotised.

Figure 2A. Image of a dead sow. Photo courtesy of Hipra.

Figure 2B. Image of a dead sow. Photo courtesy of Hipra.
Livers are enlarged and dark, and the parenchyma is uniformly infiltrated with gas bubbles, giving a spongy, “aerochocolate” appearance (Figure 3, A and B). Cases are sometimes accompanied by gastric ulcers.
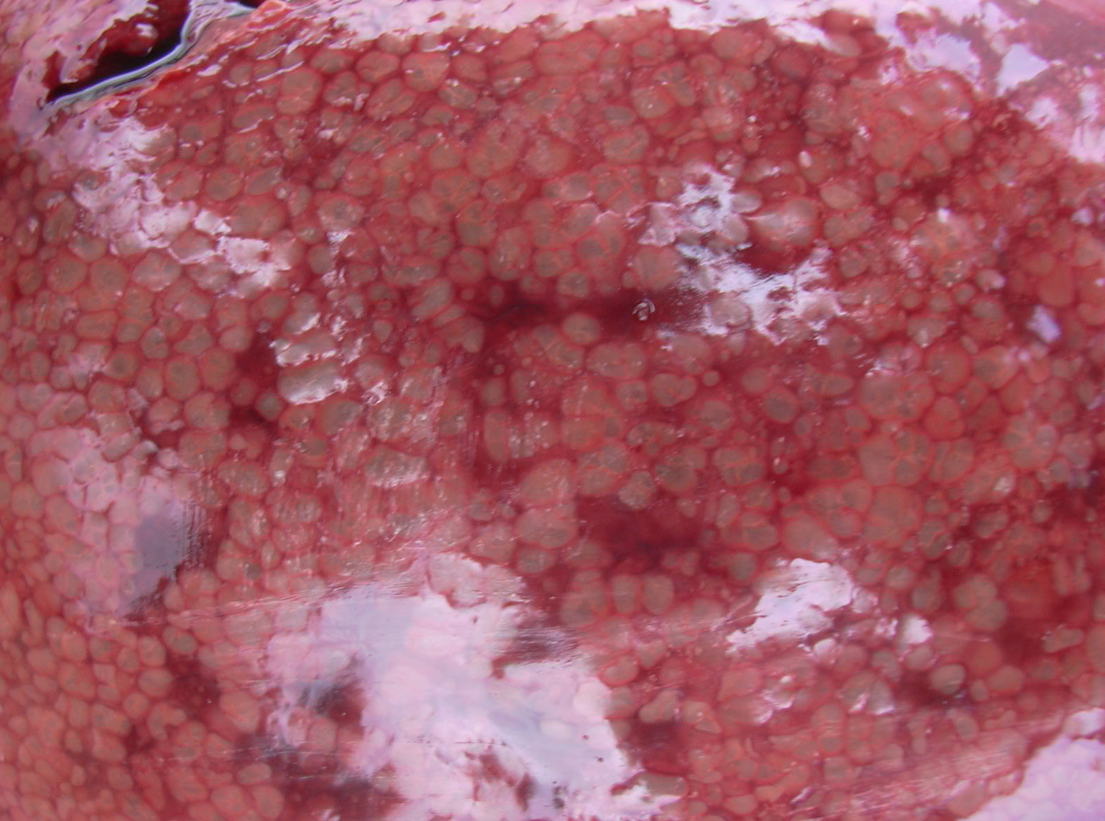
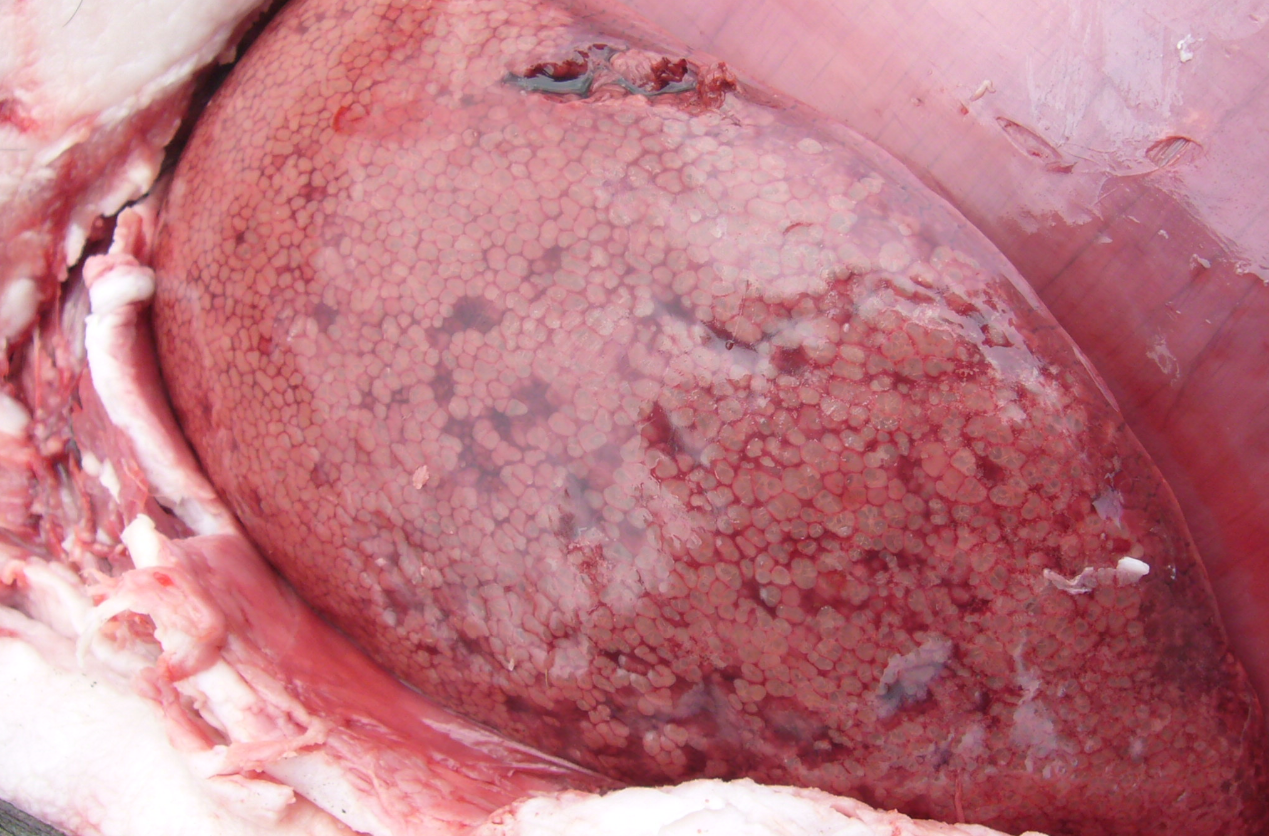
Figure 3 A,B. The liver uniformly infiltrated with gas bubbles, presenting a spongy appearance on the cut surface, probably the most distinguishing feature of sudden death in sows caused by Clostridium novyi. Photo courtesy of Dr Alfredo García Sánchez, Centro de Investigaciones Científicas y Tecnológicas de Extremadura (CICYTEX).
3. Diagnosis of C. novyi
Diagnosis of C. novyi in pigs is difficult, primarily due to the fact that in the majority of cases the animals are found dead and that the time between death and necropsy introduces the possibility of postmortem invasion. Other causes of death have to be ruled out, but it must be suspected in a case of sudden death with the necropsy findings described above (García, 2013).
3.1. Necropsy value
For accurate diagnosis, it is essential to perform postmortem examination and collect samples as soon as possible after death. Diagnosis of blackleg, malignant edema, and infections by C. novyi can be based upon clinical signs, gross and microscopic findings at necropsy (Figure 4), Gram and fluorescent antibody stains of direct smears, and bacteriologic culture.
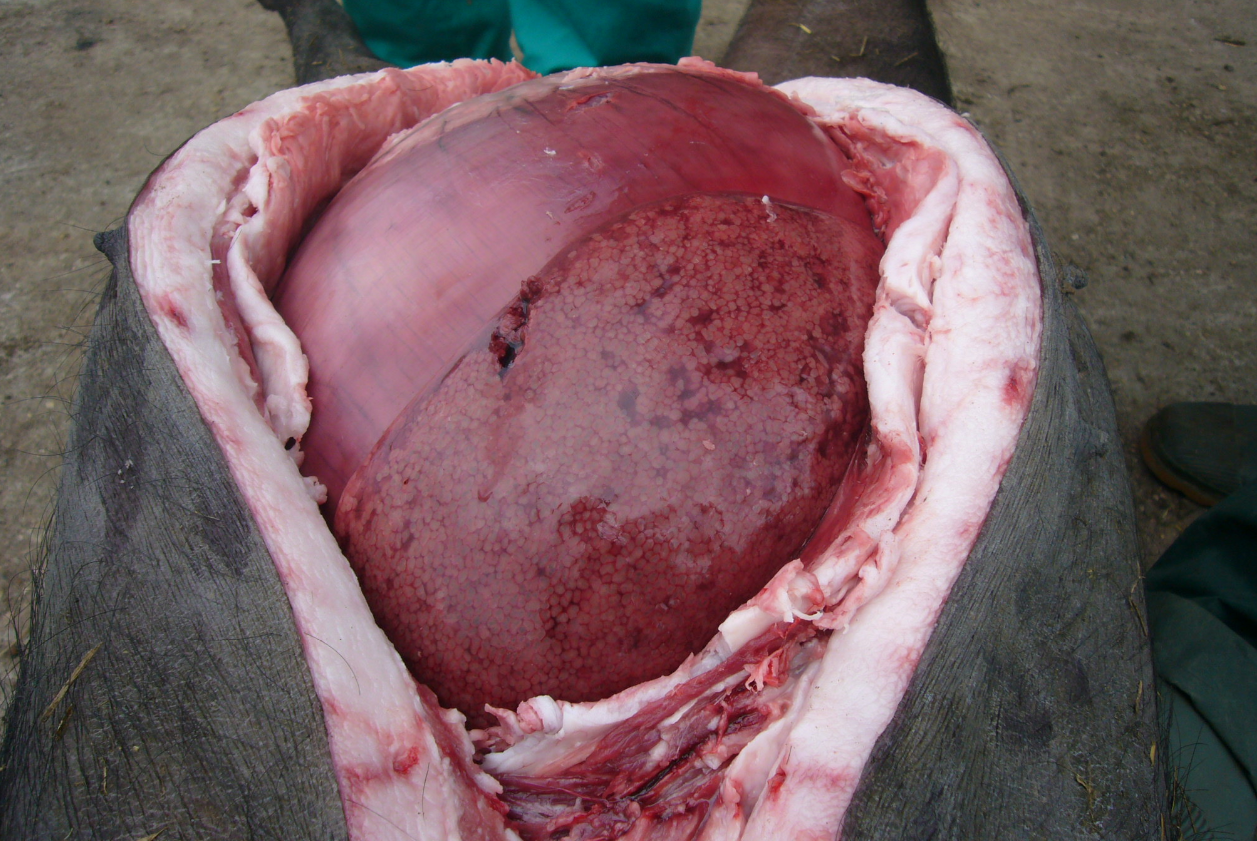
Figure 4. The abdominal cavity of a mature gestating Iberian breed sow at necropsy with enlarged and dark liver. Photo courtesy of Dr. Alfredo García Sánchez, Centro de Investigaciones Científicas y Tecnológicas de Extremadura (CICYTEX).
Colonies of C. novyi are beta hemolytic, circular, raised-to-convex, translucent, gray, and shiny, with a granular surface and erose-to-slightly scalloped margins.
3.2. Diagnostic techniques
The most useful direct and rapid differential assay for myonecrosis agents is the fluorescent antibody test. The test is performed either on smears taken directly from clinical material or on isolates. The use of direct immunofluorescence using fluorescent antibodies on smears from the liver has been described as a highly sensitive diagnostic technique, even better than culturing; however, it must be borne in mind that the longer the time between death and necropsy of the affected animals, the greater the probability of false positives (García, 2013).
Clostridia are bacteria that are difficult to culture, because they are anaerobic, with C. novyi being the most complex of those commonly found in pigs. In addition, sometimes merely isolating the clostridium species does not confirm the diagnosis, but its toxin also has to be detected as they inhabit the animal organism under normal conditions (García, 2013).
On the other hand, use of PCR procedures may allow rapid identification of C. novyi and the implicated type (Poxton, 2007; Eklund et al, 1974). In addition, enzyme immunoassay and toxin neutralization in CHO cells (Bormann and Schulze, 1999; Bormann et al, 2006) reveal alpha and beta toxins (Garcia et al, 2009), assuming that specific, toxin neutralising antibodies are available as controls.
4. Control of C. novyi
4.1. How can we control C. novyi?
Death occurs suddenly in apparently healthy animals such that, because of the speed with which the disease develops, and especially because of the role played by toxins in its development, antibiotic treatment is ineffective (McKenzie, 2012); as is well known, antibiotics act against the pathogens and not against the toxins (García, 2013).
Viable attempts to control the disease in pigs have not been conducted, other than antimicrobial prophylaxis (Post and Songer, unpublished data, 1998–present). Some practitioners in the U.S. suggest prophylaxis with bacitracin methylene disalicylate (BMD) (Anonymous, 2007). There are numerous reports in the scientific literature documenting the decrease in mortality in sows by means of the addition of bacitracin to the feed (García, 2013). Shultz (2001) observed a decrease of 16% when 250 g/ton of bacitracin methylene disalicylate was added to the diet during the last two weeks of gestation and until weaning. There was also a decrease in piglet mortality and an increase in weight gain. However in the European Union, there is currently a complete ban on the use of antibiotics as growth promoters (Regulation 1831/2003/EC on additives for use in animal nutrition).
Furthermore, the rapid elimination of carcasses by means of incineration or deep burial in lime is recommended, in order to reduce environmental contamination from the spores (García, 2013).
4.2. Prevention by means of vaccination
The brief clinical course of most histotoxic infections dictates prevention, rather than treatment, as a course of action. Commercial immunoprophylactic products usually consist of inactivated liquid cultures, eliciting antibody responses to bacterial surface antigens, toxic exoproducts, or both.
Immunisation against C. novyi-induced infectious hepatitis is routinely practiced worldwide. This type of prevention is only feasible with the use of vaccines, generally toxoids or toxoids and second-generation vaccines based on native alpha or beta or recombinant toxins (García, 2013).
All around the world, vaccines are sold which contain the alpha toxoid of C. novyi for administration to breeding sows. The manufacturers recommend a primary vaccination between 50 and 60 days antepartum and revaccination of the animals 25-30 days antepartum, with a single administration 30 days antepartum in subsequent gestations. In outbreaks of sudden death, all the animals should be vaccinated (during gestation and lactation) with revaccination 4 weeks later (García, 2013).
Based upon these findings, it seems likely that appropriately structured immunoprophylaxis may be the answer for managing the disease in swine. We would expect this disease to follow the precedent of other clostridial diseases, in which solid immunity is achieved by toxoid components in a combined clostridial toxoid.
References:
- Borrmann E and Schulze F. 1999. Detection of Clostridium novyi type B alpha toxin by cell culture systems. FEMS Immunol Med Microbiol. 24:275–280.
- Borrmann E, Schulze F, Cussler K, Hamel I, and Diller R. 2006. Development of a cell culture assay for the quantitative determination of vaccination-induced antibodies in rabbit sera against Clostridium perfringens epsilon toxin and Clostridium novyi alpha toxin. Vet Microbiol. 114:41-50
- Chen Y, Indurthi DC, Jones SW, and Papoutsakis ET. 2011. Small RNAs in the genus Clostridium. MBio. 2:e00340-10.
- Eklund MW, Poysky FT, Meyers JA, and Pelroy GA. 1974. Interspecies conversion of Clostridium botulinum type C to Clostridium novyi type A by bacteriophage. Science. 186:456-458.
- García A, Ayuso D, Benítez JM, García WL, Martínez R, and Sánchez S. 2009. Clostridium novyi infection causing sow mortality in an Iberian pig herd raised in an outdoor rearing system in Spain. J Swine Health Produc. 17:264-269.
- García A. 2013. Sanidad del ganado porcino ibérico. Principales enfermedades infecciosas. Librería Técnica Figueroa-2 (eds) PUBLICEP-Producción Humanes de Madrid, Madrid; pp 127-130.
- Hatheway CL. 1990. Toxigenic clostridia. Clin Microbiol Rev. 3:66-98.
- McKenzie E. 2012. Clostridial myositis. Large Animal Proceedings. North American Veterinary Conference, Orlando, Florida, USA, 14-18 January 2012.
- Novy F. 1894. Ein neuer anaërober Bacillus des malignen Oedems. Medical Microbiol Immunol. 17:209-232.
- Pires PS, Ecco R, de Araujo MR, Silva ROS, Salavarani FM, Heneine LGD, Assis RA, and Lobato FCF. 2012. Comparative analysis of lesions caused by histotoxic clostridia in experimentally induced myonecrosis. Semina: Ciencias Agrarias (Londrina). 33:2337-2345.
- Popoff MR and Bouvet P. 2009. Clostridial toxins. Future Microbiol. 4:1021-1064.
- Poxton IR. 2007. A PCR approach to determine the distribution of toxin genes in closely related Clostridium species: Clostridium botulinum type C and D neurotoxins and C2 toxin, and Clostridium novyi alpha toxin. J Med Microbiol. 56:196-201.
- Ryan JM, Paul J, Curtis S, and Patel NK. 2001. Clostridium novyi infection: a fatal association with injecting drug users. Emerg Med J. 18:138-139.
- Sasaki Y, Takikawa N, Kojima A, Norimatsu M, Suzuki S, and Tamura Y. 2001. Phylogenetic positions of Clostridium novyi and Clostridium haemolyticum based on 16S rDNA sequences. Int J Syst Evol Microbiol. 51: 901-904.
- Sasaki Y, Kojima A, Aoki H, Ogikubo Y, Takikawa N, and Tamura Y. 2002. Phylogenetic analysis and PCR detection of Clostridium chauvoei, Clostridium haemolyticum, Clostridium novyi types A and B, and Clostridium septicum based on the flagellin gene. Vet Microbiol. 86:257-267.
- Schranner I, Erhard MH, Kaltner H, Lapsch U. 1992. Isolation of immunogenic and lethal peptides of alpha-toxin from Clostridium novyi type B. Toxicon. 30:653-668.
- Schultz R; Dau D, Hoefling D, Duran O, Carson T, BetonL, Woodward C, Pollard K, Busker K, Kaster D, and SteidingerM. 2001. A sow mortality study- the real reasons sows die: identifying causes and implementing action. Proceedings of the American Association of Swine Veterinarians Ames, Iowa. 387-395.
- Selzer J, Hofmann F, Rex G, Wilm M, Mann M, Just I, and Aktories K. 1996. Clostridium novyi alpha-toxin-catalyzed incorporation of GlcNAc into Rho subfamily proteins. J Biol Chem. 271:25173-25177.
- Songer JG. 1996. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 9:216-234.







