



Pharmacokinetic and Pharmacodynamic Relationships of Tiamulin (Denagard®) for Enteric and Respiratory Infections in Pigs
Pharmacokinetic (PK) and pharmacodynamic (PD) integration has become one of the cornerstones for understanding how antibiotics work against bacteria, according to Novartis Animal Health.Introduction
As our knowledge improves and our understanding develops the application of PK/PD integration can be extended into drug/effect modelling and dose estimation in early research and development, to dose confirmatory assessment for regulatory affairs and practical applications for veterinarians in selecting effective dose rates for both clinical efficacy, even eradication programmes and resistance development control.
Each drug has its own PK profile and PD activity, usually measured as minimum inhibitory concentration (MIC) and it is the purpose of this paper to review the activity of tiamulin hydrogen fumarate (Denagard®; Novartis Animal Health Inc.) against enteric infections such as swine dysentery caused by Brachyspira hyodysenteriae, porcine intestinal spirochaetosis ‘colitis’ caused by B. pilosicoli and porcine proliferative enteropathy ‘ileitis’ caused by Lawsonia intracellularis. On the respiratory side, the PK/PD relationships of tiamulin and Mycoplasma hyopneumoniae (enzootic pneumonia) and Actinobacillus pleuropneumoniae will be examined.
Tiamulin Pharmacokinetics
Tiamulin injection pharmacokinetics
Tiamulin when injected displays its own special distribution and range of PK concentrations in various tissues and potential infection sites (McKellar et al., 2004) (see Figure 1).
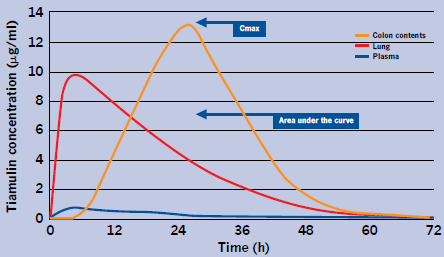
Tiamulin achieves relatively low levels in plasma in comparison with concentrations in the lung. The maximum concentration (Cmax) is 15.7 times higher in lung and the area under the curve (AUC) lung is 18.1 times higher than the AUC plasma. The colon contents Cmax is 21 times higher than plasma. The drug is excreted via the bile into the gut contents and concentrates in the colon. This gives tiamulin its special qualities of treating both enteric and respiratory infections.
Tiamulin oral pharmacokinetics
When given orally, tiamulin is well absorbed, metabolised in the liver and excreted in the bile. The plasma levels are quite low because of this but again it concentrates in lung tissue as it is lipid-soluble and has a good cell penetration and distribution. As tiamulin is excreted in the bile, it joins the gut contents, with whatever was not absorbed originally, and then passes through the ileum, bathing the ileal wall, where L. intracellularis primarily colonises and then concentrates in the colon. The ileum concentration is approximately 25 to 30 per cent of the colon concentration (Burch, 2005), and Novartis's own estimation is 29 per cent.
The concentration of tiamulin in lung, plasma (estimated), colon and ileum (estimated) contents following oral administration via the drinking water and via feed as measured by Anderson et al., (1994) (see Table 1).
| Table 1. Tiamulin oral pharmacokinetics (Anderson et al., 1994) | ||
| Tiamulin dose rate in water | 13.2mg/kg bwt 120ppm tiamulin | 20.9mg/kg bwt 180ppm tiamulin |
|---|---|---|
| Tiamulin lung conc. (μg/g) | 4.3 | 8.5 |
| Tiamulin plasma conc. E (μg/ml) | 0.24 | 0.47 |
| Tiamulin colon contents (μg/g) | 5.59 | 18.58 |
| Tiamulin ileum contents E (μg/g) | 1.62 | 5.39 |
| Tiamulin dose rate In feed | 13.2mg/kg bwt 220ppm tiamulin | |
| Tiamulin lung conc. (μg/g) | 1.99 | |
| Tiamulin plasma conc. E (μg/ml) | 0.11 | |
| Tiamulin colon contents (μg/g) | 8.1 | |
| Tiamulin ileum contents E (μg/g) | 2.4 | |
| Key: E = Estimated; Ileum concentration = 29% of colon contents concentration; Plasma concentration = lung concentration / 18.1 | ||
Tiamulin is better absorbed from drinking water – the same dose achieves approximately double the lung concentration. When given in feed, higher tiamulin concentrations are achieved in the gut.
Tiamulin Pharmacodynamics
A number of recent studies have been carried out to look at the susceptibility of pathogenic bacteria to tiamulin. The pharmacodynamics are usually expressed as the MIC50, MIC90 and range of MICs (see Table 2).
| Table 2. Tiamulin MIC50, MIC90 and MIC range for various organisms | ||||
| Reference (source) | Organism (no. of isolates) | MIC50 (μg/ml) | MIC90 (μg/ml) | Range (μg/ml) |
|---|---|---|---|---|
| Wattanaphansak et al., 2009 (EU, US) | L. intracellularis (10) | ≤0.125 | ≤0.125 | ≤0.125 |
| Adachi et al., 2008 (J) | B. hyodysenteriae (77) | 0.2 | 1.6 | <0.1-12.5 |
| Pridmore, 2008 (EU) | B. pilosicoli (33) | 0.062 | 4.0 | 0.008-4.0 |
| Kobayashi et al., 2008 (J) | M. hyopneumoniae (90) | 0.06 | 0.125 | ≤0.03-0.125 |
| Fodor et al., 2004 (H) | A. pleuropneumoniae (10) | 2 | 4 | 2.0-4.0 |
| VetPath II, 2009 (EU)* | A. pleuropneumoniae (10) | 8 | 16 | 0.25-16 |
| Key: * = CLSI method; EU = European; J = Japanese; H = Hungarian concentration = lung concentration / 18.1 | ||||
Tiamulin is very active against L. intracellularis with all the intracellular MICs at or below 0.125µg/ml. B. hyodysenteriae and B. pilosicoli are also highly susceptible but there are a number of isolates with high MICs. M. hyopneumoniae generally is inhibited by very low MICs of tiamulin, but conversely the MICs against A. pleuropneumoniae are very high, suggesting that a different PK/PD relationship is involved other than plasma concentration.
Treatment and Prevention of Ileitis
The PK/PD relationships of tiamulin and L. intracellularis are demonstrated in Figure 2.
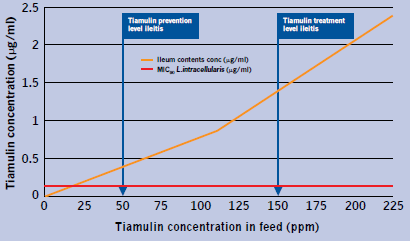
These results suggest there should be good inhibition of L. intracellularis at 50ppm tiamulin and a very strong treatment effect at 150ppm with ileum content concentration at three and 11 times the MIC, respectively.
This was confirmed in an ileitis infection study (McOrist et al. 1996) when pigs were artificially challenged and given a prevention dose of 50ppm Denagard for 21 days from infection and 150ppm Denagard for 14 days, seven days after infection. No gross or histopathological lesions were detected in the treated groups. The intracellular MIC of the organism used was 0.125µg/ml (see Table 3).
| Table 3. Treatment and prevention of ileitis with tiamulin in an artificial challenge study | |||
| Treatment group | Gross lesions pigs affected (%) | Histological lesion score ileum (%) | Histological lesion score caecum (%) |
|---|---|---|---|
| Infected untreated control | 86 | 42 | 46 |
| Prevention: Denagard 50ppm from -2 to 21 days | 0 | 0 | 0 |
| Treatment: Denagard 150ppm 7-21 days | 0 | 0 | 0 |
Treatment of Swine Dysentery
In an artificial infection study (Taylor, 1982), tiamulin was used at in-feed dosage of 0, 50, 80, 120 and 160ppm for 14 days. The MIC of the isolate used was 0.5µg/ml. A definite dose response could be seen with a bactericidal effect observed at approximately six times the MIC with 120ppm and above (see Table 4).
| Table 4. Treatment of swine dysentery with tiamulin in an artificial challenge study | ||
| Denagard in feed (ppm) Day 0-14 | Swine dysentery (clinical & bacteriological) day 0 | Re-isolation of B. hyodysenteriae day 14 |
|---|---|---|
| 0 | 3/5 | 3/5 |
| 50 | 2/5 | 1/5 |
| 80 | 2/5 | 1/5 |
| 120 | 4/5 | 0/5 |
| 160 | 3/5 | 0/5 |
Enzootic Pneumonia
Anderson et al. (1994) demonstrated that tiamulin given in drinking water gives a much higher lung and plasma concentration than when given in the feed (see Figure 3).

Two separate artificial infection studies looked at the use of tiamulin for the treatment of M. hyopneumoniae.
Trial 1 – (Underdahl and Szanto, 1976). Gnotobiotic piglets were treated seven days after infection for five days using tiamulin at 4.4 and 8.8mg/kg bodyweight administered twice daily via the milk. Pigs were necropsied 21 days post-infection and the MIC of the organism was 0.1µg/ml.
Trial 2 – (Hannan et al., 1982). Pigs were treated at 10mg/kg bodyweight twice daily for 10 days, 14 days after they had been infected with lung homogenate and necropsied 38 days after infection.
The trial was repeated twice and the MICs of the strains re-isolated were 0.1 to 0.25µg/ml (see Table 5).
| Table 5. Combined enzootic pneumonia trial results – lung lesion scores (%) | ||||
| Trial | Denagard 0 mg/kg | Denagard 8.8 mg/kg | Denagard 17.6 mg/kg | Denagard 20 mg/kg |
|---|---|---|---|---|
| 1 | 100 | 100 | 21 (-79%) | - |
| 2a | 100 | - | - | 22 (-78%) |
| 2b | 100 | - | - | 2 (-98%) |
At the higher dose rates there was a substantial reduction in lesions (78 to 98 per cent) indicating a bactericidal effect at approximately three times the MIC.
Actinobacillus pleuropneumoniae
In an artificial infection study (Schultz & Anderson, 1983), pigs were infected intranasally with A. pleuropneumoniae (ST5) with an MIC of 4.0µg/ml. At the first signs of clinical pneumonia they were treated with Denagard in the drinking water at 0, 60, 120 and 180ppm (approximately 6, 12, 18mg/kg bodyweight) for five days and the surviving pigs were necropsied 21 days PI (see Table 6).
| Table 6. Results of A. pleuropneumoniae challenge study | ||||
| Denagard (ppm) |
Mortality (24h) |
Ave lung lesion score (%) | Ave lung lesion score (%), live pigs | App re-isolation |
|---|---|---|---|---|
| 0 | 2/8 | 100 | 100 | 7/8 |
| 60 | 1/8 | 100 | 92 | 6/8 |
| 120 | 1/8 | 52 | 19 | 1/8 |
| 180 | 0/8 | 2 | 2 | 0/8 |
Tiamulin at 120ppm showed an intermediate effect against A. pleuropneumoniae but 180ppm showed a bactericidal effect in spite of a strong challenge. Interestingly, the minimum bactericidal concentrations (MBCs) for tiamulin are approximately double the MICs (Pridmore et al., 2010) and fit in very well with lung concentrations as the PK parameter rather than plasma concentrations. It has also been demonstrated that tiamulin concentrates in polymorphs to a similar plasma concentration ratio (Nielsen and Szancer, 1998) and it is considered that this is a likely explanation for efficacy.
Conclusions
Tiamulin has unique pharmacokinetics for both respiratory and enteric infections. The antibiotic is well absorbed from the gut and goes via the plasma to concentrate in lung tissue and probably macrophages and neutrophils. Excretion is via the bile so bioactive residues and parent compound is also re-excreted into the gut and concentrates in the ileum and colonic contents.
Plasma concentrations are normally associated with efficacy against M. hyopneumoniae and administration via the water gives higher lung and plasma levels of tiamulin than via the feed. High concentrations are highly effective against A. pleuropneumoniae.
Administration via the feed is thought to slow the absorption of tiamulin and reduce its bioavailability but higher concentrations are achieved in the gut when given in feed. Tiamulin is very active and effective via this route against L. intracellularis the cause of ileitis, B. hyodysenteriae the cause of swine dysentery and B. pilosicoli, the cause of colitis in pigs.
References
- Adachi, Y. et al. 2008. Proceedings of the 20th IPVS Congress, Durban, S. Africa, vol.2:239.
- Anderson, M.D. et al. 1994 Proceedings of the AASP Congress, Chicago, Illinois, USA, 115-118.
- Burch, D.G.S. 2005. Pig Journal, 56:25-44.
- Fodor, L. et al. 2004. Proceedings of the 18th IPVS Congress, Hamburg, Germany, vol.2:563.
- Hannan, P. et al. 1982. Research in Veterinary Science, 33:76-88.
- Kobayashi, H. et al. 2008. Proceedings of the 20th IPVS Congress, Durban, S. Africa, vol.2:187.
- McKellar, Q.A. et al. 2004. Proceedings of the 18th IPVS Congress, Hamburg, Germany, vol.2:622.
- McOrist, S. et al. 1996. Veterinary Record, 139:615-618.
- Nielsen, B.H. and Szancer, J. 1998. Proceedings of the 15th IPVS Congress, Birmingham, UK, 3:241.
- Pridmore, A. 2008. Report to Novartis.
- Pridmore, A. et al. 2010. Proceedings of the AAVM Congress, Tel Aviv, Israel. In press.
- Schultz, R. and Anderson, M. 1983. Diamond Shamrock Report.
- Taylor, D.J. 1982). Proceedings of the 7th IPVS Congress, Mexico City, Mexico, p47.
- Underdahl, N. and Szanto, J. 1976. Squibb Report.
- VetPath II (2004-2006) Report. 2009. ‘Determination of the antimicrobial susceptibility of VetPath II (2004-2006) collection of bacterial pathogens.’
- Wattanaphansak, S. et al. 2009. Veterinary Microbiology, 134:305-310
Further Reading
| - | Find out more information on the diseases mentioned in this article by clicking here. |
February 2012






