



ProBe-Bac® SE: a new preventive approach for bacterial infection
Bacteriophages as an aternative to antibioticsMultiple approaches have been utilized to prevent and treat swine enteric colibacillosis in an effort to promote health and growth performance, with antibiotics being the most commonly applied option (Castro et al., 2022). Therefore, the development of the antimicrobial resistance (AMR) has reduced treatment options for pig producers and raised public health concerns about the potential transfer of AMR genetic determinants into the food chain, water, and manure, among other areas, as a result of the growing selective pressure of antibiotic use to treat these Escherichia coli infections (Barros et al., 2023). It is crucial to take into account that E. coli has a strong capacity for acquiring resistance genes, primarily by horizontal gene transfer, in which mobile genetic elements appear to play a significant role in the transmission.
Are there alternatives to antibiotics?
Antibiotic-resistant bacteria are rapidly emerging worldwide, which compromises the efficiency of antimicrobials to transform medicine and save millions of lives. According to the Global Antibiotic Research and Development Partnership (GARDP), the discovery of antibiotics has transformed the world by curing diseases that were formerly fatal. The catastrophe caused by AMR has been associated with misuse or overuse of these medical treatments. Meanwhile, the One Health approach integrates multiple sectors to put regulations in place to prevent devastating AMR outbreaks. The Organization for Economic Co-operation and Development (OECD) recommends alternatives to antimicrobials which are safe and sustainable such as probiotics, prebiotics, and bacteriophages that could reduce infection, thus improving animal health (OECD, 2022).
Bacteriophages as safe and sustainable alternative to antibiotics
Worldwide multidrug resistance entails the implementation of alternative way to control infections. The use of bacteriophages is one of the strategies and highly recommended in reducing and eliminating harmful bacteria in animal production (Alomari et al., 2021). Bacteriophages have attracted much attention as a potential alternative solution considering the reduced efficacy of antibiotics due to AMR. Additionally, Zbikowska et al. (2020) stated the majority of studies have focused on the effectiveness of bacteriophages in reducing bacterial counts and in the management of bacterial infections in animals, which are zoonotic and substantially impact public health.
Bacteriophage infects bacteria by adhering to the bacterial cell membrane and injecting its genetic material into the bacterial host. Bacteriophages then multiply through the bacterial host machinery, which causes bacterial cell rupture. Compared to antibiotic which can be non-selective, bacteriophages have excellent specificity to target hosts. They could specifically recognize and attack only pathogenic bacteria, and do not harm humans or other living organisms. This is in agreement with the findings of Upadhaya et al. (2021) that bacteriophages are specialized for certain bacteria, and because they only infect one species, serotype, or strain, phage therapy has been reported to be safe and effective in comparison to antibiotics. The proliferation of commensal intestinal bacteria is not hampered by this mechanism of action. By reducing particular pathogenic microbial populations while promoting the proliferation of beneficial microbiota, the use of bacteriophages as a feed additive may be able to offer an integrated strategy for modulating the gut microbiome in animals, leading to improved gut health.
The U. S. Food and Drug Administration (FDA) has confirmed the safety of using bacteriophages, and had approved as Generally Recognized as Safe (GRAS) substance. Henceforth, Pathway Intermediates, together with Optipharm, its affiliate company leading in the field of animal diagnosis and biomedical research, is exerting efforts for the development and commercialization of a new bacteriophage solution called ProBe-Bac.
ProBe-Bac: Pathway Intermediates’ bacteriophage solution
ProBe-Bac is the latest bacteriophages solution of Pathway Intermediates. It is a cocktail product which contains a powerful mixture of bacteriophages precisely selected against specific disease. The newly developed ProBe-Bac has improved stability and coverage which maximizes the efficiency in eliminating bacterial pathogens. Furthermore, ProBe-Bac SE is one version of the product specifically designed for swine targeting enteric bacterial diseases such as edema, colibacillosis, salmonellosis, and diarrhea. The bacteriophages in ProBe-Bac would settle in the animal intestine upon ingestion, which selectively kills pathogenic bacteria in a specific-manner, improves intestinal environment, and enhances feed efficiency.
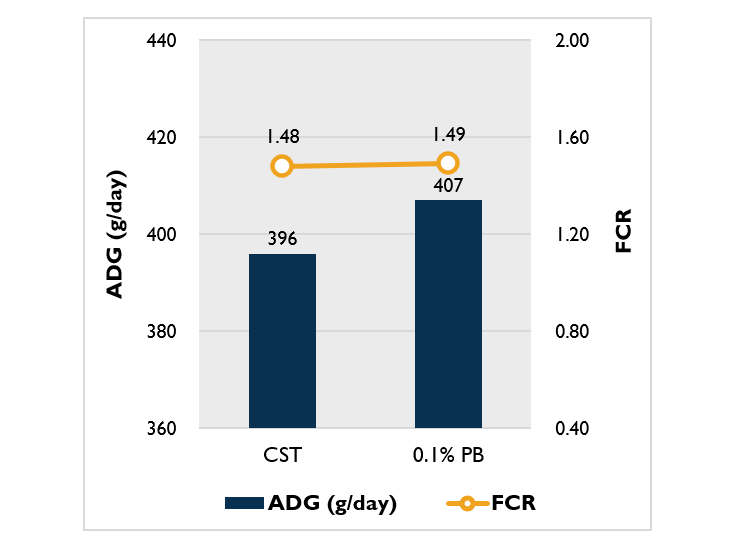
Effects of ProBe-Bac SE on growth performance of piglets
A recently published study by Kingkan et al. (2023) has investigated the beneficial effects of ProBe-Bac SE on the growth performance of piglets. The investigation was carried out with 800 weanling piglets. The treatments included CST (basal diet + 160 ppm Colistin) and 0.1% PB (basal diet + 0.1% ProBe-Bac SE).
The feeding trial revealed an improved average daily gain (ADG) for the animal group that received 0.1% inclusion level of ProBe-Bac SE (Figure 1). FCR was not different between the two treatments, which indicates that ProBe-Bac SE could be an alternative to antibiotics. Promisingly, the overall result was remarkable, revealing that ProBe-Bac SE supplementation improved growth performance of weaned piglets, and evidently even better than antibiotic treatment.
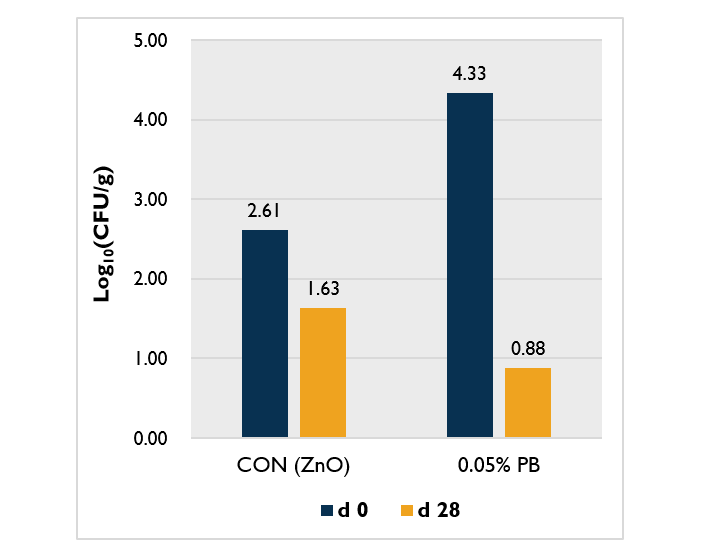
Effects of ProBe-Bac SE on fecal bacterial population of post-weaning pigs
Another study was performed on a commercial farm in Thailand to investigate the effect of supplementation of ProBe-Bac SE in reducing the fecal bacterial population in pigs compared to zinc oxide, which is commonly used alternative to antibiotics. A total of 2,000 post-weaning pigs were randomly allocated to two dietary treatments: CON (commercial diet with zinc oxide) and 0.05% PB (commercial diet without zinc oxide + 0.05% ProBe-Bac SE).
After 28 days of dietary inclusion of ProBe-Bac SE to the diet of post weaning pigs, the population of Enterotoxigenic Escherichia coli F18 (ETEC F18) decreased by 80% indicating a better effect compared to zinc oxide treatment (Figure 2). This finding suggests that bacteria may have developed defense mechanisms and resistance to antimicrobials. Meanwhile, ProBe-Bac SE, the latest bacteriophage solution that specifically recognizes and attacks the target bacterial host, could be recommended as a substitute for antibiotics that contribute to the growth promotion of pigs.
Conclusion
The results revealed that dietary supplementation of ProBe-Bac SE improved the growth performance of weaned piglets, demonstrating an even better effect than antibiotic treatment. Additionally, the decrease in population of ETEC F18 signifies the excellent effectiveness of ProBe-Bac SE compared to other dietary treatment. Collectively, these findings suggest that ProBe-Bac SE could be considered as a promising alternative to antibiotics, contributing to growth promotion and improvement of the intestinal microflora in pigs.
For more information, please contact your Pathway Intermediates representative.






