



Reduction of nasal lesions and production losses after vaccination against non-progressive atrophic rhinitis
A new study demonstrates the efficacy of vaccination against NPAR.Background and objectives
The primary agent of non-progressive atrophic rhinitis (NPAR), Bordetella bronchiseptica (Bb), has a high prevalence worldwide1. Currently, vaccination is regarded as one of the alternative methods for the prevention of bacterial diseases2. The aim of the study was to demonstrate the efficacy of an inactivated vaccine against NPAR based on the reduction of nasal lesions and production losses in piglets during lactation and the nursery period.
Materials and methods
A French farrow-to-finish pig herd with respiratory symptoms caused by Bb during nursery was selected for a controlled clinical trial. Six pregnant sows were primo-vaccinated with RHINISENG® (V Group) following the manufacturer’s instructions, while six others received phosphate-buffered saline solution (PBS) (NV Group). The parameters evaluated in these sows’ piglets were NPAR nasal lesions3,4 at 43 days of age and individual body weight during lactation and the nursery period.
Results
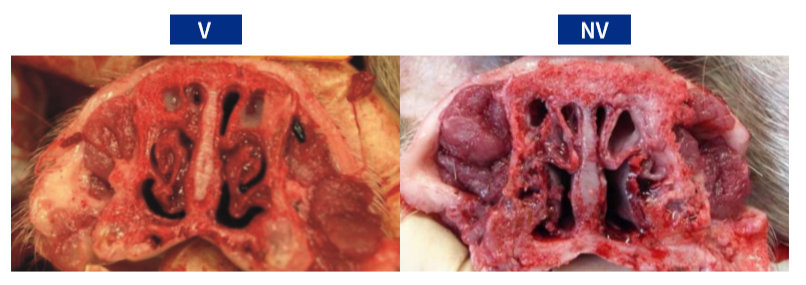
The Bb agent was confirmed at the herd selection and during the trial. Piglets from the NV Group presented significantly higher mean nasal lesion scores than the V Group (3.17 vs. 1.67) (Table 1; figure 1).
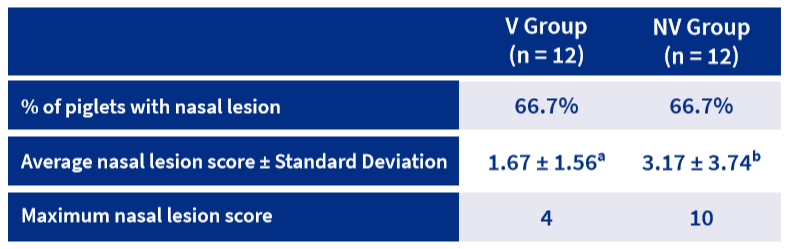
*a,b: Statistically significant differences (P values <0.01) at the same parameter are indicated with defferent superscripts.
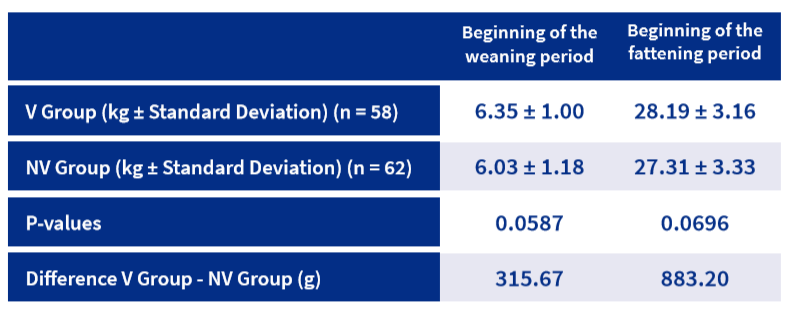
Regarding production parameters, mean body weight per piglet tended to be higher in the V Group both at weaning (6.35 kg vs. 6.03 kg) and at the end of the nursery period (28.19 kg vs. 27.31 kg) (Table 2).
Discussion and conclusion
These results show that immunisation of pregnant sows with RHINISENG® prior to farrowing allowed to protect piglets against NPAR by reducing nasal lesions associated with the disease. Moreover, vaccination showed a tendency to improve body weight; the larger size of the studied population may have improved the statistical significance of the results. These confirm similar experiences with the vaccine in previous trials5.
Acknowledgements
The authors wish to thank Guilhem Poudevigne, Olivier Maniaval, the Laboratoire Départamental d'Analyses du Tarn and the UCAM staff for their technical support.
| References | ||||
|---|---|---|---|---|
| 1. Hammond, EE et al. | ||||
| (2009) | . Zoo Wildl Med | 40:369-372 | ||
| 2. Comission notice | ||||
| (2015) | . official Journal of the European Union | 2015/C 299/04 | ||
| 3. Magyar, T et al. | ||||
| (2002) | Evaluation of vaccines for atrophic rhinitis - a comparison of three challenge models.. Vaccine | 20: 1797-1802 | ||
| 4. European pharmacopeia 8.0 | ||||
| (2004) | . Monograph | 01/2004:1361 | ||
| 5. Sanchez-Matamoros, A et al. | ||||
| (2017) | Field efficacy study of RHINISENG® in Europe in a farm with problems associated with Bordetella bronchiseptia.. AAASV |







